Calculate the number of grams of solute that has to be used to make the following solution: 216.5 mL of 0.116 M HCI g HCI eTextbook and Media Hint Save for Later Last saved 1 day ago. At Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes.
Calculate the number of grams of solute that has to be used to make the following solution: 216.5 mL of 0.116 M HCI g HCI eTextbook and Media Hint Save for Later Last saved 1 day ago. At Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 60QAP: An aqueous solution of M2O is prepared by dissolving 10.91 g of the electrolyte in 435 mL of...
Related questions
Question
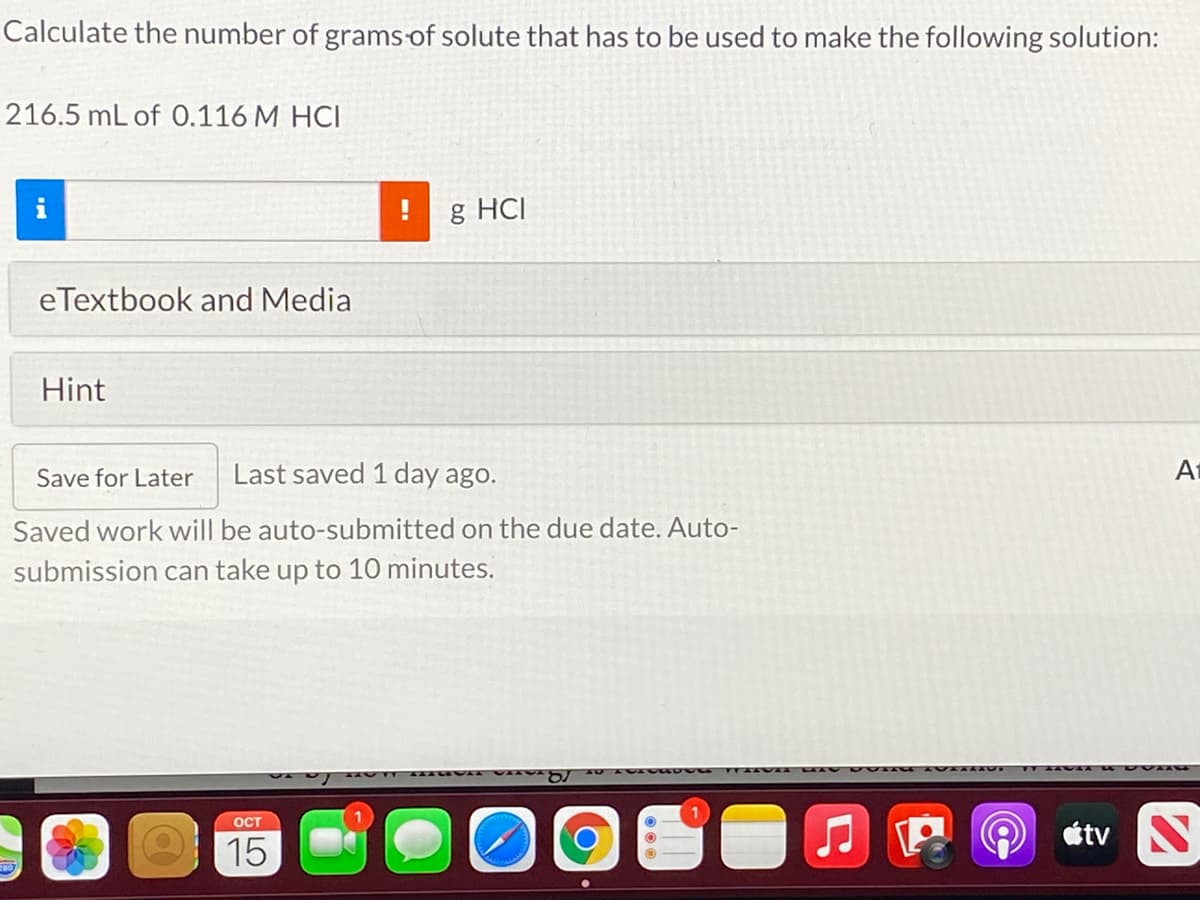
Transcribed Image Text:Calculate the number of grams of solute that has to be used to make the following solution:
216.5 mL of 0.116 M HCI
g HCI
eTextbook and Media
Hint
Save for Later
Last saved 1 day ago.
At
Saved work will be auto-submitted on the due date. Auto-
submission can take up to 10 minutes.
OCT
15
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning