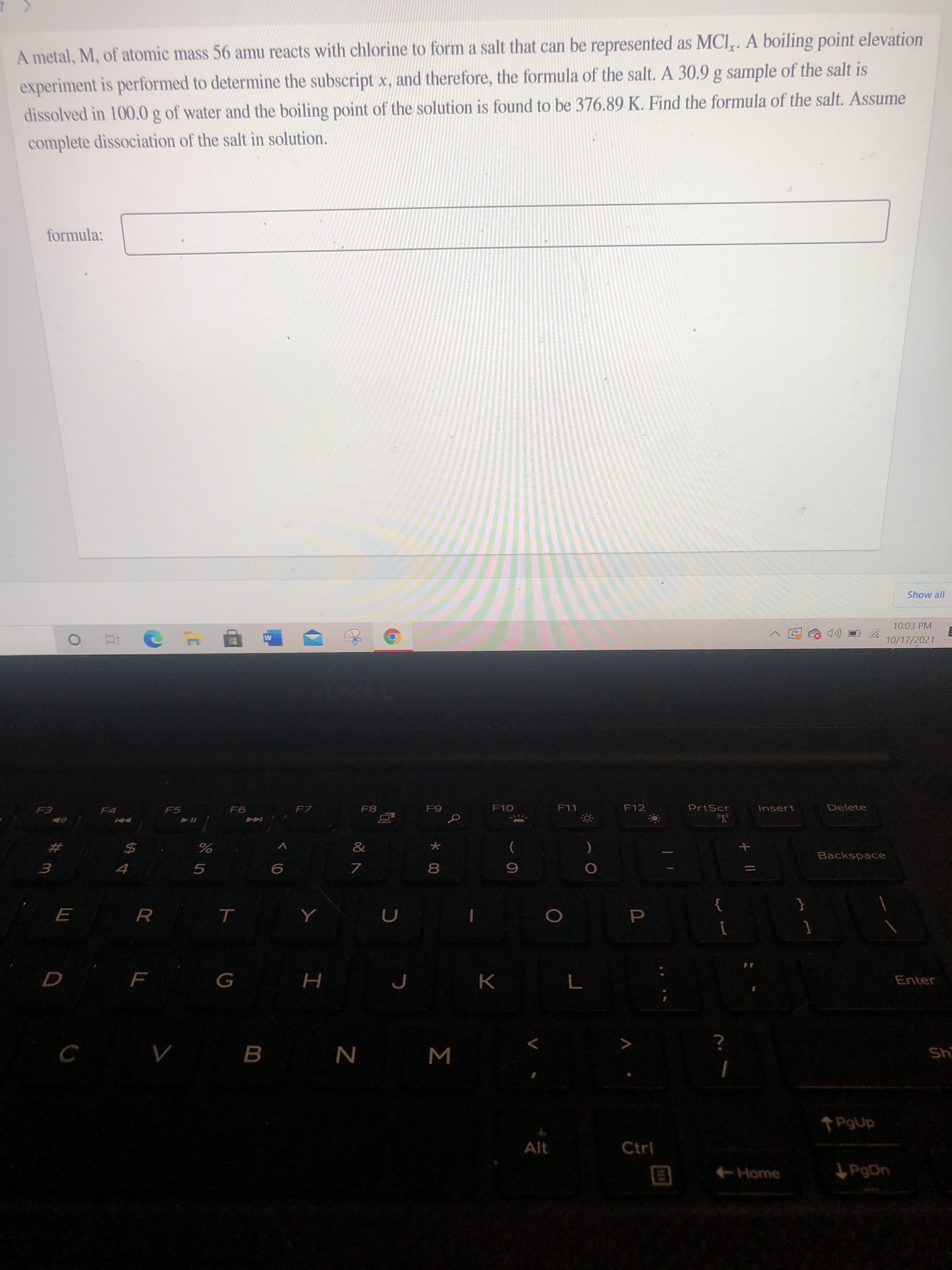
A metal, M, of atomic mass 56 amu reacts with chlorine to form a salt that can be represented as MCI,. A boiling point elevation experiment is performed to determine the subscript x, and therefore, the formula of the salt. A 30.9 g sample of the salt is dissolved in 100.0 g of water and the boiling point of the solution is found to be 376.89 K. Find the formula of the salt. Assume complete dissociation of the salt in solution. formula:
A metal, M, of atomic mass 56 amu reacts with chlorine to form a salt that can be represented as MCI,. A boiling point elevation experiment is performed to determine the subscript x, and therefore, the formula of the salt. A 30.9 g sample of the salt is dissolved in 100.0 g of water and the boiling point of the solution is found to be 376.89 K. Find the formula of the salt. Assume complete dissociation of the salt in solution. formula:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 76AP
Related questions
Question
Transcribed Image Text:Σ
A metal, M, of atomic mass 56 amu reacts with chlorine to form a salt that can be represented as MCI,. A boiling point elevation
experiment is performed to determine the subscript x, and therefore, the formula of the salt. A 30.9 g sample of the salt is
dissolved in 100.0 g of water and the boiling point of the solution is found to be 376.89 K. Find the formula of the salt. Assume
complete dissociation of the salt in solution.
formula:
Show all
10:03 PM
10/17/2021
F5
F6
F7
F8
F10
F11
F12
PrtScr
Delete
Insert
%23
%24
)
Backspace
5.
8.
R.
Enter
H
B.
us
N
Alt
Ctrl
Home
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole