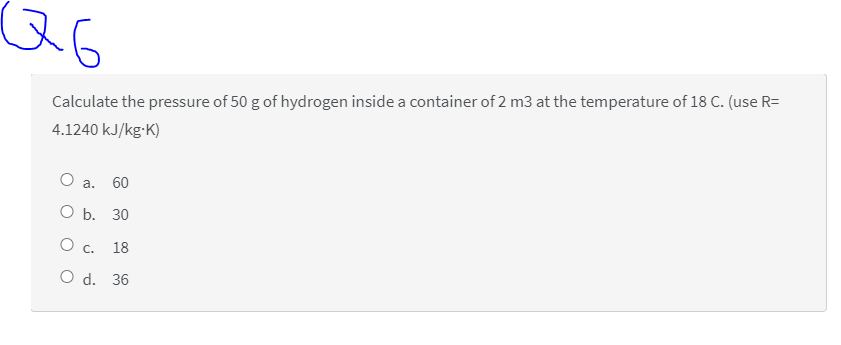
Calculate the pressure of 50 g of hydrogen inside a container of 2 m3 at the temperature of 18 C. (use R= 4.1240 kJ/kg•K) О а. 60 ОБ. 30 O c. 18 O d. 36
Calculate the pressure of 50 g of hydrogen inside a container of 2 m3 at the temperature of 18 C. (use R= 4.1240 kJ/kg•K) О а. 60 ОБ. 30 O c. 18 O d. 36
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter1: Heat, Temperature, And Pressure
Section: Chapter Questions
Problem 6RQ: One British thermal unit will raise the temperature of _____ 1b of water _____F.
Related questions
Question
Transcribed Image Text:QG
Calculate the pressure of 50 g of hydrogen inside a container of 2 m3 at the temperature of 18 C. (use R=
4.1240 kJ/kg-K)
O a.
60
ОБ. 30
О с. 18
O d. 36
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning