Calculate the solubility (in grams per 1.00 x 102 mL of solution) of magnesium hydroxide in a solution buffered at pH = 12. Express your answer using two significant figures. V ΑΣφ S1 = g/ (1.00 x 102 mL) Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part B How does this compare to the solubility of Mg(OH)2 in pure water? Express your answer using two significant figures. IνΟ ΑΣφ S1 Submit Request Answer
Calculate the solubility (in grams per 1.00 x 102 mL of solution) of magnesium hydroxide in a solution buffered at pH = 12. Express your answer using two significant figures. V ΑΣφ S1 = g/ (1.00 x 102 mL) Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part B How does this compare to the solubility of Mg(OH)2 in pure water? Express your answer using two significant figures. IνΟ ΑΣφ S1 Submit Request Answer
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.54QE
Related questions
Question
Idk how to solve this. Can someone help me? I'm also super confused on how to convert the L thing to 1.0*10^2ml.
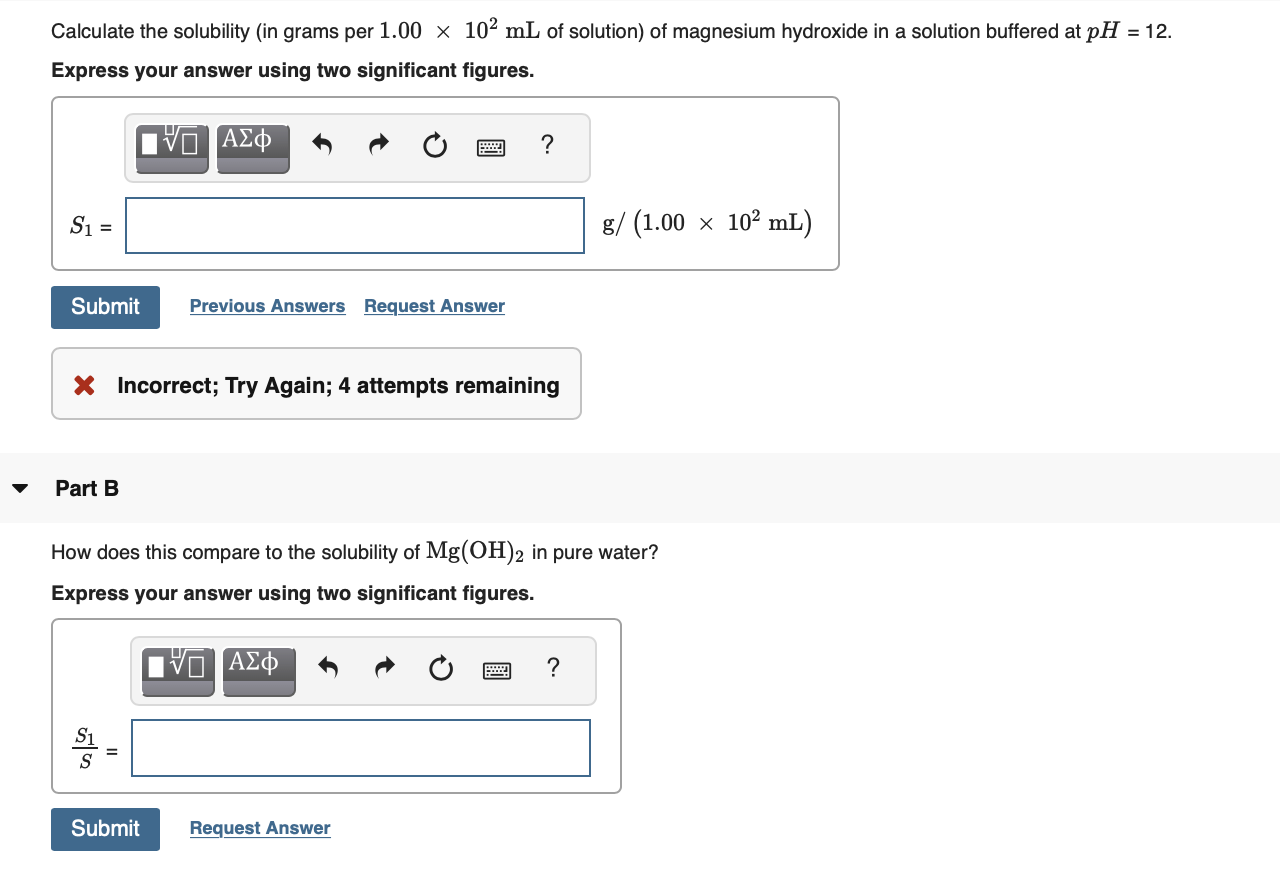
Transcribed Image Text:Calculate the solubility (in grams per 1.00 x 102 mL of solution) of magnesium hydroxide in a solution buffered at pH = 12.
Express your answer using two significant figures.
V ΑΣφ
S1 =
g/ (1.00 x 102 mL)
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Part B
How does this compare to the solubility of Mg(OH)2 in pure water?
Express your answer using two significant figures.
IνΟ ΑΣφ
S1
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning