Calculate the standard free energy change for each of the following metabolically important enzyme-catalyzed reactions, using the equilibrium constants given for the reactions at 25°C and pH 7.0 a. Glutamate + oxaloacetate aspartate + aketoglutarate enz. Apartate aminotransferase; K'eg = 6.8 b. Fructose 6-phopshate + ATP E fructose 1,6-bisphosphate + ADP enz. Triose phosphate isomerase; K'eg =254 wwww
Calculate the standard free energy change for each of the following metabolically important enzyme-catalyzed reactions, using the equilibrium constants given for the reactions at 25°C and pH 7.0 a. Glutamate + oxaloacetate aspartate + aketoglutarate enz. Apartate aminotransferase; K'eg = 6.8 b. Fructose 6-phopshate + ATP E fructose 1,6-bisphosphate + ADP enz. Triose phosphate isomerase; K'eg =254 wwww
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 13P
Related questions
Question
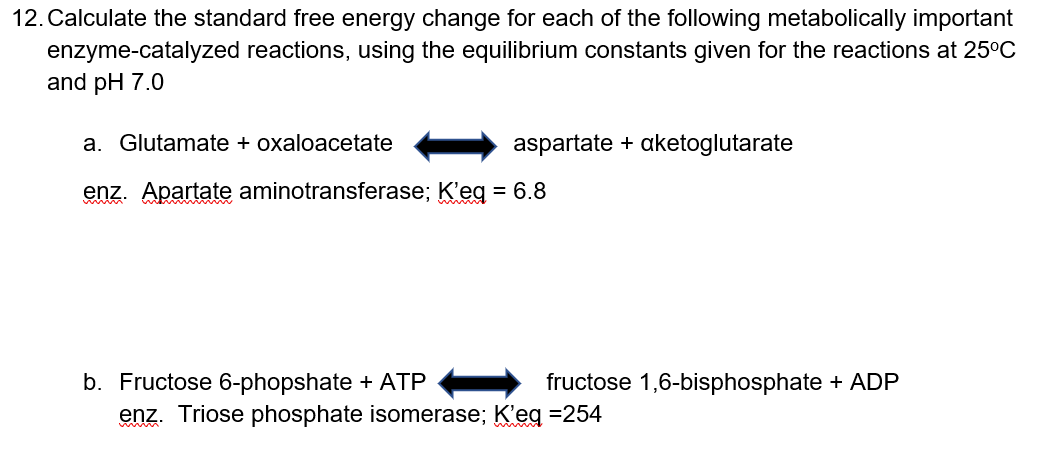
Transcribed Image Text:12. Calculate the standard free energy change for each of the following metabolically important
enzyme-catalyzed reactions, using the equilibrium constants given for the reactions at 25°C
and pH 7.0
a. Glutamate + oxaloacetate
aspartate + aketoglutarate
enz. Apartate aminotransferase; K'eg = 6.8
b. Fructose 6-phopshate + ATP
- fructose 1,6-bisphosphate + ADP
enz. Triose phosphate isomerase; K'eg =254
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning