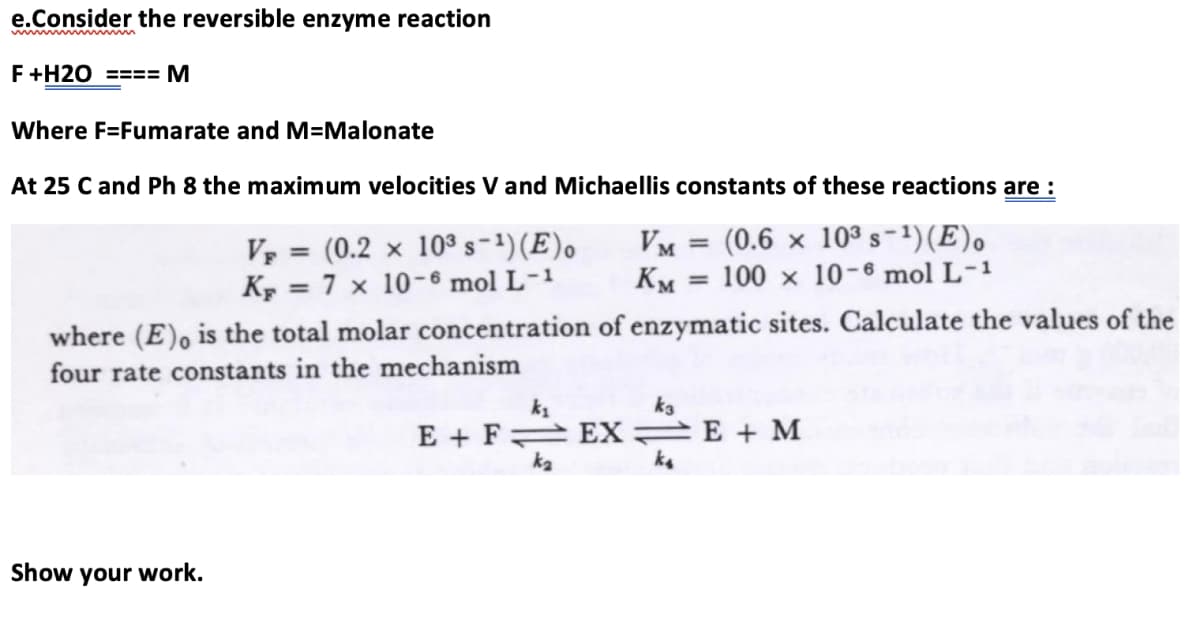
Calculate the values of the
Q: Which of the following statements are TRUE about eukaryotes? (i) They are cells with a nucleus. (ii)...
A: Cell is the structural and functional unit of life. It can be classified into prokaryotic and eukary...
Q: 50 Which best describes the role of FADH2 in aerobic metabolism? O FADH2 is a proton shuttle and tra...
A: Metabolism includes biosynthesis/ reduction (an anabolic process) and oxidation (catabolic p...
Q: why is there glucose 6-phospI n the ER lumen A. Glycogenolysis occurs in the ER lumen. B. It prevent...
A: Introduction: Glycogenolysis is the catabolism of glycogen to form glucose. The glycogen branches ar...
Q: What is meant by resistant? Give the mechanism by which organisms develop resistance.
A: Antibiotics can be classified based on the mechanism of action. These functions include the antimicr...
Q: Given the following reaction, identify the class and subclass of the enzyme involved. CH2OH C=O H-C-...
A: Enzymes catalyse the reaction either by making or breaking the bond. They might form double bonds or...
Q: AP is a 35-yr old male presents with hypertension. His medications were known to work by inhibiting ...
A: Any of several naturally occurring amines that act as neurotransmitters and hormones in the body is ...
Q: Failed to follow
A: Waxes are a broad category of organic compounds that are lipophilic and bendable solids at room temp...
Q: Which of the polysaccharides WILL DECREASE GELLING if acid is added to the sample? pectins ...
A: Gels are solid, jelly-like structures made of colloid polysaccharides, proteins, and polymers produc...
Q: Lysine is similar to ornithine. .Why does lysine not form a lactam? a. Infrequency of lysine occurr...
A: A lactam is a cyclic amide (lactone+amide). A lactam antibiotic is an antibiotic that contains a β-l...
Q: Other than oxidative phosphorylation, what other metabolic pathway does "Complex 2" participate in? ...
A: Oxidative phosphorylation is the process by which ATP is synthesized as a result of electron transpo...
Q: The transcription of a gene called YFG (your favoritegene) is activated when three transcription fac...
A: An enhanceosome is a group of trans-acting factors that assemble at an enhancer region of a gene to ...
Q: Among these amino acid combinations listed above, only the combination of Lys and Glu have side chai...
A: The tertiary structure of proteins are stabilized by non covalent interactions like hydrogen bonding...
Q: estion properly and accordingl
A: The saponification value of oil is measured by the number of milligrams of potassium hydroxide (KOH)...
Q: Branched polysaccharides will be more viscous than linear polysaccharides. True False
A: The molecular formula of glucose is C6H12O6. Glucose is a building block of disaccharides like ...
Q: If you have to prepare 4 nM solution of human IgG ( immunoglobuline) which has molecular mass of 150...
A: The number of moles of a solute present in one liter of the solution is called the molarity of that ...
Q: is an amino acid that can be directly converted into a citric acid cycle intermediate by being expos...
A: Citric acid cycle is the final common oxidative pathway for carbs, proteins and lipid. Amino acids ...
Q: Using G-25 Sephadex beads (Fractionation range 1000-5000) KD, a sample of Vitamin B12 (MW ~1500 KD) ...
A: Note : Hi ! Thank you for the question. We are authorized to answer one question at a time. Since yo...
Q: Identifies the roles of ventilation and PCO₂ in acid/base balance . Answer ASAP .
A: Acid is present in body in stomach.Acid and base balence in body is important for human health.Acid ...
Q: Which of the following is a drawback of using base hydrolysis during amino acid composition? A. Pro...
A: The amino acids that occur naturally as constituents of proteins have an amino group (NH2) and a car...
Q: Just as all life shares a last universal common ancestor,all eukaryotes share a last eukaryotic comm...
A: The most recent common ancestor of all existing life on Earth, also known as the last universal comm...
Q: Which of the following statements regarding size exclusion chromatography is false? During siz...
A: Hi! Thank you for the question, as per the honor code, we are allowed to answer the first...
Q: Concanavalin (ConA) is a 25.5KDa protein with pl of 4.7 and optical absorbance (A 0.1% 289) of 1.14....
A: Proteins are polymers of amino acids with specific molecular weight and pI (isoelectric point, pH at...
Q: I. Kwashiorkor, also known as «cdematous malnutrition» because of its association with edema (fluid ...
A: Kwashiorkor or Edematous malnutrition: Kwashiorkor is a protein nutritional disorder associated with...
Q: 3. Draw out the first 3 enzymatic reactions of the PPP, including listing names of S, P, coenzymes, ...
A: Hi! Since you have posted multiple questions and have not mentioned which to answer, we are answerin...
Q: 1.The 22nd amino acids and the only amino acid * A. Selenocysteine B. Selenicysteine C. Carbocystei...
A: Since you have asked multiple questions, we will solve only first question for you. If you want any ...
Q: How many H2O particles were produced by creating the following structure? * R H R R H-N-C,-c- -N-C,-...
A: Amino acids are monomers of protein they are linked with each other by forming peptide...
Q: Analysis of a octapeptide revealed the presence of the following products: 2 Arg, 1 Gly, 1 Met, 1 Tr...
A: Edman Degradation – This is method of peptide sequencing. In this method amino terminal residue is l...
Q: Which statement(s) correctly describe(s) protein structures? A. All hydrophobic residues are buried ...
A: Proteins are made up of amino acids, which are the building blocks. Around 20 different amino acids ...
Q: Which of the following is INCORRECTLY paired? O Isoelectric focusing : Charge O Gel filtration chrom...
A: 1. Isoelectric focusing IEF is an electrophoretic method for separating proteins based on their isoe...
Q: Show the relationship between lipids, nucleic acids, proteins, and carbohydrates including the conne...
A: A biomolecule, also called a biological molecule, is a chemical compound found in living organisms. ...
Q: Match the outcomes on the left with the laboratory steps on the right. ___ Harvest the cells ...
A: Introduction: DNA isolation is a process of purification of DNA from samples using a combination of ...
Q: Double-helical DNA has major and minor grooves because
A: The question is regarding DNA double helix structure. The structure of a DNA molecule is described a...
Q: Both choices B and D are correct.
A: Cholic acid which is also called as 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid and represents as a p...
Q: What is insulin resistance? Hypoglycemia? Hyperglycemia? Explain its similarities and differences
A: Insulin is a peptide hormone produced by the beta-cells of islets of Langerhans of the pancreas. It ...
Q: For the ion concentrations in Table 12.1, calculate the equilibriumpotentials for each ion species i...
A: ions can move in either direction through a channel (i.e., either into or out of the cell) the d...
Q: Biochemistry Question: Nonenzymatic glycosylation or glycation creates glycoproteins by
A: What is glycoproteins: Glycoproteins are molecules that comprise protein and carbohydrate chains tha...
Q: In Figure 12-26, provide a biochemical mechanism forwhy HP-1 can bind to the DNA only on the left si...
A: The family of heterochromatin protein 1 (HP-1) consists of highly conserved proteins which perform a...
Q: Why is stereochemistry of biomolecules important in biological processes?
A: Stereochemistry is the Study of three dimensional structure of molecules and their effect on the fun...
Q: 6. DNA electrophoresis uses polyacrylamide gel for separation. a) True b) False 7. Agarose is a natu...
A: DNA is composed of nucleotides attached via phosphodiester bonds. DNA act as genetic material in mos...
Q: Using a semi-permeable membrane, dialysis allows the removal of salt ions prior to chromatography. T...
A: Dialysis is a separation process that uses selective and passive diffusion via a semi-permeable memb...
Q: Suppose you want to determine if excessive lipid ingestion altersgene transcription. Describe and ex...
A: Dietary fat is a crucial macronutrient for the increase and development of all organisms. In additio...
Q: Some mice have a mutation in the liver isozyme of pyruvate kinase leading to an inability to be phos...
A: Pyruvatw kinase in an enzyme which is involved in the glycolysis process. In Glycolysis, the cycle A...
Q: hat happened to the DNA at the different temperatures (95, 75, 55 degrees)? How does Polymerase Chai...
A: DNA is a double stranded polynucleotide chain ,the double stranded structure is formed by the comple...
Q: (a) A solution containing these five proteins was adjusted to pH 7.0 and then applied to a SIZE-EXCL...
A: Chromatography is a method of separation of specific compounds from the mixture of compounds. This c...
Q: Give one example of 5-Carben Sugar
A: A sugar is a polyalcohol that has had at least one of its constituent alcohols oxidized to an aldehy...
Q: Discuss about enzymes: function, definition, and examples.
A: The human body is made up of various types of cells, tissues, and other complicated organs. To maint...
Q: What is the purpose of DNA methylation? Why is it ineffective in the DNA repair of spontaneous deami...
A: DNA methylation is the process of adding methyl groups to DNA at Nucleotide bases, so that without c...
Q: Match the following structural composition of each polysaccharide with its identity ...
A: Polysaccharides, also known as polycarbohydrates, are the most abundant carbohydrates found in food....
Q: Biochemistry Question: Nonenzymatic glycosylation or glycation creates glycoproteins by
A: Glycoproteins are molecules that comprise protein and carbohydrate chains that are involved in many ...
Q: Which of the following cause and effect relationships below is incorrect? higher ligand bindi...
A: Which of the following cause and effect relationships below is incorrect? higher ligand binding: in...
Step by step
Solved in 2 steps

- Utilising the provided class data generate the following graphs: I) Michaelis Menten; II) Lineweaver-Burk; and III) Hanes-Woolf. Ensure that you clearly label each graph,and add the relevant trendlines with equations. Table 1: Class data demonstrating the Absorbance at 700nm obtained for the alkaline phosphatase enzyme reaction Table 1 tube Abs700mm 1 0.000 2 0.060 2 0.090 4 0.140 5 0.190 6 0.250 7 0.290 The equipment we used are • 20mM Tris Buffer pH 8.5 • 33mM MgCl2 • Alkaline Phosphatase (2mg/ml) in 20mM Tris Buffer pH 8.5 • 4mM Glucose-1-phosphate • Acid Molybdate pH 5.0 • Reducing Agent • Distilled Water • Glass Test tubes • Tube Rack • Cuvette • Pipettes and Tips • Water bath set to 37oC The method we used is Method/Protocol: 1. Read the protocol in its entirety before starting. Take note of any additional information that appears in subsequent steps that may influence how previous steps are performed. 2. Using glass tubes, generate the reactions mixtures…Consider the following equilibrium at 25ºC :Glucose-1-Phosphate Glucose-6-PhophateUsing the equilibrium concentrations of [Glucose-1-Phosphate] = 0.35 M and [Glucose-6-Phosphate] = 1.65 M, calculate BOTH K′eqand Gº′ for this reaction. Is this reaction exergonicor endergonic? R = 8.314 J/K·molGiven the following information, calculate the catalytic efficiency of the enzyme. Step by step please [S] = 100 mM k1 = 10 sec-1 k2 = 3000 sec-1 k-1 = 20 sec-1 [E]T = 1 \muμM
- a) Calculate the enzyme and specific activity of a reaction with 3 μM Hsp90 using the following information: The rate is measured in a spectrophotometer as 0.028 OD units/min in a 1 ml reaction volume. The absorbance was detected at 340nm and the extinction coefficient for NADH at this wavelength is 6200 L M-1 min-1 and the molecular mass of Hsp90 is 82.7 kDa. The rate of NADH utilisation is equivalent to the rate of ATP utilised by Hsp90. Show all your calculations and the units for your answers. b) Calculate the turnover number for the reaction described in (a) aboveBased on the definition of kcat, substitute a value that can be measured and yet still represents the value associated with the original concentration of the R. What would the rate or velocity of the reaction be equal to under these circumstances? How can cells increase Vmax? What variable that we could change would directly impact Vmax? Would the value of KM be affected by the ways you determined that Vma,x could be increased? What does this indicate about KM? Thinking about how catalysts work, about the Michaelis-Menten Equation, and the definition of kcat, what specifically does the enzyme change in the reaction mechanism to increase the rate? If an enzyme follows the 2 step mechanism proposed by Michaelis-Menten, what do you know about this enzyme? Be very specific and comprehensive. Please answer very soon will give rating surelyCalculate the equilibrium concentration of H2O for the following esterification reaction performed in ethanol(C2H5OH) C2H5OH + CH3CO2H ⇌CH3CO2C2H5 + H2O KC= 4.0 At equilibrium: [CH3CO2H] = 0.75 M; [CH3CO2C2H5 ]= 2.2 M
- A) Is this reaction ( in picture provided) in equilibrium? B) If it is not then ,what is ∆G' at 25°C if the concentration of Glucose-1-phosphate is 15.04µM and the concentration of Glucose-6-phosphate is 1.62 mM? Answer in Joules. Round to the correct number of significant figures. (There are 103 µM in 1mM.) Thank you so Much!!!The conversion of glucose-1-phosphate to glucose-6-phosphate by the enzyme phosphoglucomutase has a △G°' of -7.6 kJ/mol. Calculate the equilibrium constant for this reaction at 298 K and a pH of 7. (R = 8.315 J/K-mol) A. 0.003 B. 0.047 C. 1.00 D. 21With appropriate chemical structures, explain the mechanism (mode-of-action) of fluoroacetate poisoning? Example: Step 1: Fluoroacetate is converted to Product “A”. This reaction is catalyzed by Enzyme __________________________ Structures of fluoroacetate and the product “A”. Name of Enzyme. Step 2: Product “A” from Step 1 is converted to Product “B.” Catalyzed by enzyme 2. Structure of Product B and name of Enzyme 2. etc.
- The formation of maltose, a disaccharide, from two glucose molecules, is not energetically favorable. However, if this reaction is coupled with the hydrolysis of ATP, the reaction occurs more favorably. Maltose + H2O = 2 Glucose , ΔG'o = -15.5 KJ/mol or -3.7 kcal/mol a. Determine if the coupled reaction will occur spontaneously at standard state through calculating the Gibbs Free Energy of Reaction. b. Calculate the equilibrium constant for each individual reaction, and for the coupled reaction (using free energy of reaction). Show that the equilibrium constant for the coupled reaction equals the equilibrium constants for the individual reactions multiplied together. c. If the reaction medium contains the following chemical species at their given concentrations (298 K and 1.0 atm, pH = 7.0), will the reaction proceed in the forward or the reverse direction? [Maltose] = [Glucose] = 10.0 mM; [ATP] = 5.0 mM; [ADP] = [Pi] = 20 mMIf a 0.1 M solution of glucose 1- phosphate at 25 °C is incubated with a catalytic amount of phosphoglucomutase, the glucose 1-phosphate is transformed to glucose 6-phosphate. At equilibrium, the concentrations of the reaction components are Calculate Keq and ΔG′° for this reaction.From your Lineweaver-Burk plot,the vlaues are: Km Vmax Uninhibited 0.09 mmol/L 3.02 min/mmol Inhibited 6.22 mmol/L 9.98 min/mmol By describing the potential changes in the kinetic parameters, identify and justify the type of inhibitor that was inhibiting the acid phosphatase activity.

