Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.6QAP
Related questions
Question
Practice Pack
Transcribed Image Text:- 5.1 Dropoff Point
E CH 5.1: Spectra Exercise Sheet
locs.google.com document/d/1K1BK4hXg9trgR1iFzCeQDqsclpudai6kiszwDsfmtyE/edit
"ouTube
* spanish
VOL Community service.
M Gmail
Notebooks
* Whiteboard chemis.
Flipgrid | 828488ga
Ani
ctra Exercise Sheet
Request edit access
w Tools Help
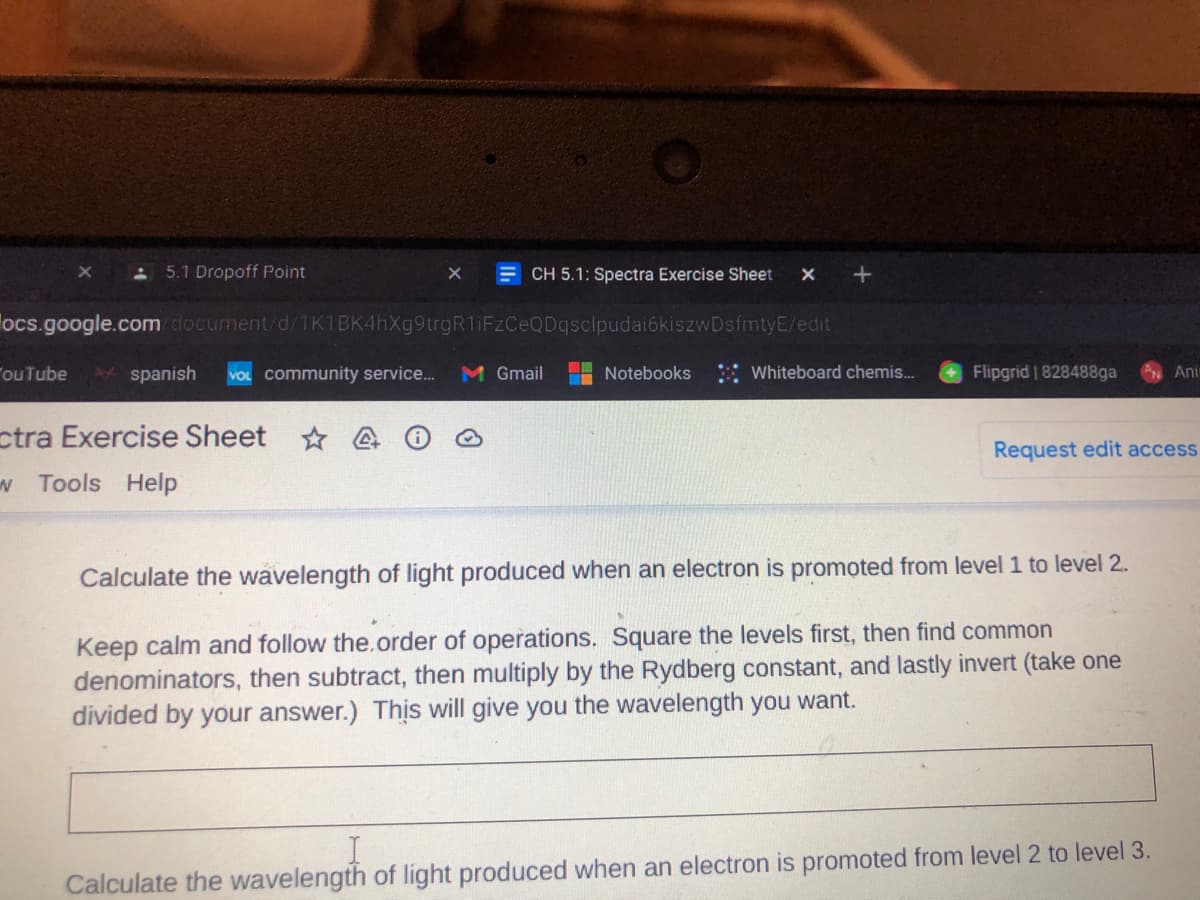
Calculate the wavelength of light produced when an electron is promoted from level 1 to level 2.
Keep calm and follow the.order of operations. Square the levels first, then find common
denominators, then subtract, then multiply by the Rydberg constant, and lastly invert (take one
divided by your answer.) This will give you the wavelength you want.
Calculate the wavelength of light produced when an electron is promoted from level 2 to level 3.
Transcribed Image Text:A 5.1 Dropoff Point
E CH 5.1: Spectra Exercise Sheet
+
i docs.google.com document/d/1K1BK4hXg9trgR1iFzCeQDqsclpudai6kiszwDsfmtyE/edit
D YouTube
* spanish
VOL community service...
M Gmail
Notebooks
: Whiteboard chemis..
Flipgrid | 828488ga
Anim
: Spectra Exercise Sheet
Request edit access
dit View Tools Help
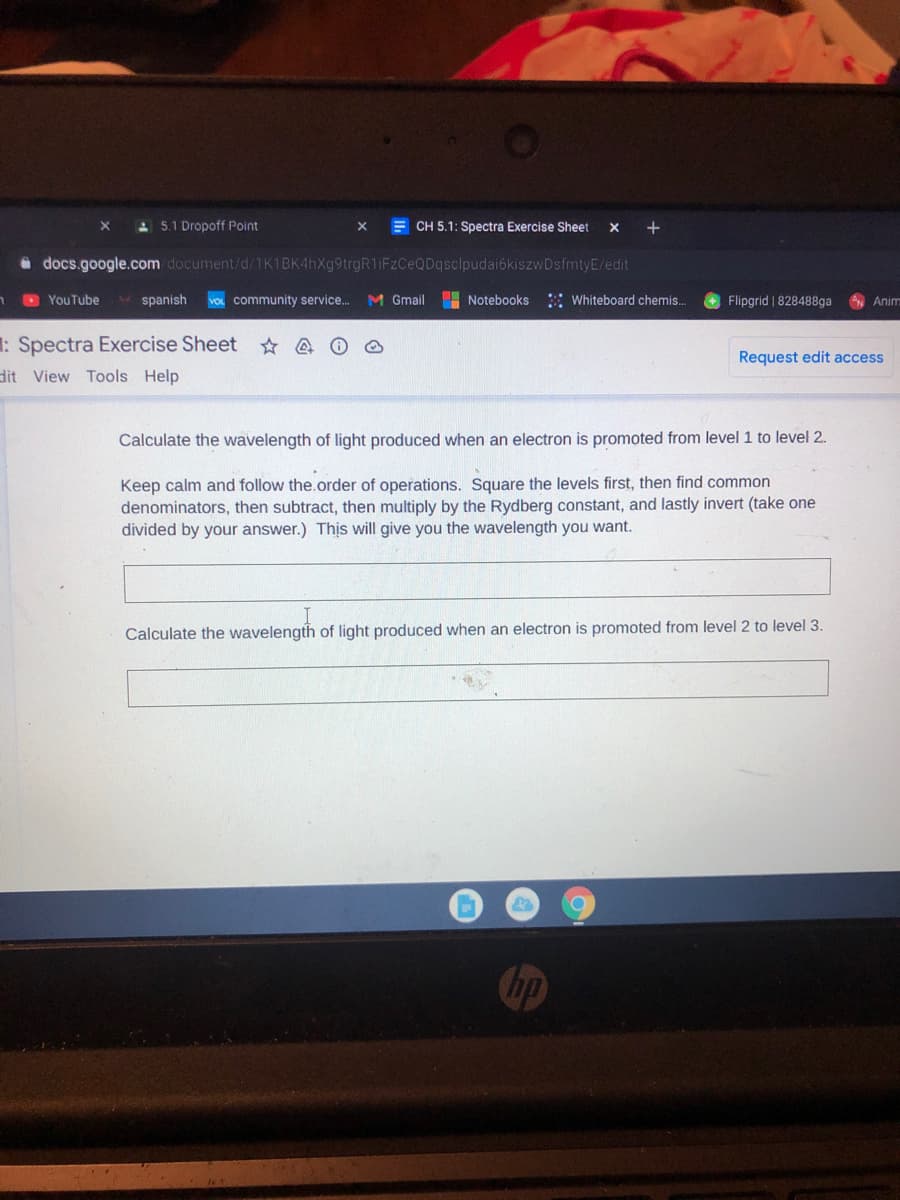
Calculate the wavelength of light produced when an electron is promoted from level 1 to level 2.
Keep calm and follow the.order of operations. Square the levels first, then find common
denominators, then subtract, then multiply by the Rydberg constant, and lastly invert (take one
divided by your answer.) Thịs will give you the wavelength you want.
Calculate the wavelength of light produced when an electron is promoted from level 2 to level 3.
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Includes step-by-step video
Learn your way
Includes step-by-step video
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
