Calculate the wavelength pf light that is absorbed when a n electron in the molecule CH2=CH-CH=CH-CH=CH-CH=CH-CH=CH2 is promoted from the HOMO to the LUMO. The average bond length can be taken to be 144 pm. SHOW ALL YOUR WORK, INCLUDING HOW YOU CHOSE THE HOMO AND LUMO.
Calculate the wavelength pf light that is absorbed when a n electron in the molecule CH2=CH-CH=CH-CH=CH-CH=CH-CH=CH2 is promoted from the HOMO to the LUMO. The average bond length can be taken to be 144 pm. SHOW ALL YOUR WORK, INCLUDING HOW YOU CHOSE THE HOMO AND LUMO.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 41P
Related questions
Question
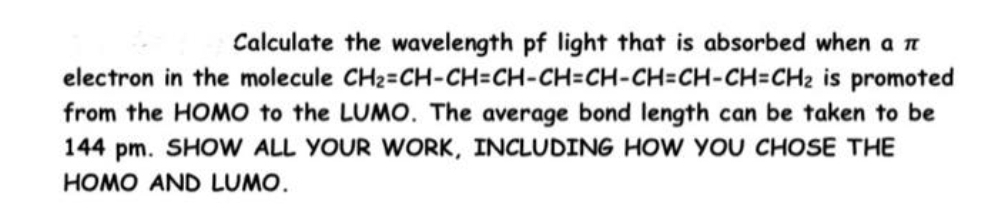
Transcribed Image Text:Calculate the wavelength pf light that is absorbed when a n
electron in the molecule CH2=CH-CH=CH-CH=CH-CH=CH-CH=CH2 is promoted
from the HOMO to the LUMO. The average bond length can be taken to be
144 pm. SHOW ALL YOUR WORK, INCLUDING HOW YOU CHOSE THE
HOMO AND LUMO.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,