Q: You took 25.00 mL of unknown water solution and titrated it with EDTA. The EDTA solution molarity…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: EDTA is one of the common reagents used in a complexometric titration. a. Can we use EDTA…
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and…
Q: 0.75 gm of calcium carbonate is dissolved in 1 liter of distilled water. 100 ml of this standard…
A: 0.75 gm of calcium carbonate is dissolved in 1 liter of distilled water. 100 ml of this standard…
Q: Considering the graph given, what is the most suitable pH for the titration of calcium with EDTA?…
A: pH 10 buffer is used in EDTA titration because in EDTA Y4- is predominant, and we want Y4- to react…
Q: 50.00 mL of a solution containing iron (II) and iron (III) when titrated at pH2.0, 12.50 mL 0.01200…
A: Answer is explained below. From the given plot it is clear that at ph = 2.0, only Fe3+ reacts with…
Q: Compare the two quantities based on the given condition: At the equivalence point in the titration…
A: At the equivalence point, all of the Ca2+(aq) is converted to CaY2-(aq).
Q: 5. 20 mL of tap water sample is taken in a conical flask and 1 mL of pH 10 buffer and 3 drops of…
A: Hard water is the water that contains dissolved Ca2+ and Mg2+ ions. These ions react with fatty…
Q: An EDTA solution was prepared by dissolving approximately 4 g of the disodium salt in approximately…
A: Given mass of MgCO3 = 0.07682 g Volume of water = 1 L Molar mass of MgCO3 = 84.31 g/mol
Q: Using the graph given, which metal can be titrated better with EDTA? A. Fe3 + B. Fe2 + C. Ca2 + D.…
A: pH 10 buffer is used in EDTA titration because in EDTA Y4- is predominant, and we want Y4- to react…
Q: Which of the following causes the sharpest change in the equivalence point of a complexometric…
A: A multiple choice question based on complexometric titration, which is to be accomplished.
Q: When doing a titration, it is helpful to know approximately the volume of titrant that will be…
A: The equation to calculate the unknown value from known value is given by, M1V1=M2V2 where M is the…
Q: which of the following is the concentration of the EDTA solution in terms of molarity?
A: Ca2+ forms a complex compound with EDTA ligand. EDTA is a hexadentate ligand. Onle mole of Ca2+…
Q: If 10.00 mL sample of 0.01 M standard Ca2+ solution is titrated with 9.8 mL of EDTA solution, what…
A: Interpretation - To determine the molarity of the EDTA when 10.00 mL sample of 0.01 M standard Ca2+…
Q: 20. Assume you are using 0.1 M EDTA to titrate a 0.2 M Ca* solution (25 mL) at pH 10.0. V molarity…
A: Calculation of no. of mol of Ca2+: n=Molarity×Volume=0.2 mol/L×25×10-3 L=5×10-3 mol Calculation of…
Q: A student titrated a 25.00 mL sample of water with 0.0200 M EDTA. The titration required 20.40 mL of…
A: This is a simple volumetric titration, using M1V1=M2V2 we can obtain the strength of water sample.…
Q: Write the mass balance equation for EDTA and the complete charge balance for the complexometric…
A: Various titrations are studied in chemistry. One of them is complexation titration. It takes place…
Q: Considering the graph given, what is the most suitable pH for the titration of calcium with EDTA?
A: The EDTA titration of Ca2+ ions is carried out at high pH values. An increase in pH enhances the…
Q: Using the graph given, which metal can be titrated better with EDTA? A. Fe3+ B. Fe2+ C. Ca2+ D.…
A: From the graph we clearly see that complex formation constant of Fe3+ is higher among all the given…
Q: Elaborate the reason why EDTA is used in many complexometric titrations. How will you be able to…
A: complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the…
Q: Which of the following is completely true regarding the titration of iron oxalate salt with EDTA? It…
A: In inroganic laboratories, Titrations are carried out to find out the concentrations of an unknown…
Q: The total concentration of Ca²+ and Mg²+ in a sample of hard water was determined by titrating a…
A: SOLUTION: Step 1: The concentration of magnesium ion is calculated as follows:
Q: 50.00 mL of a solution containing both Ni2* and Pb²+ ions requires 46.32 ml of a 0.02041 M EDTA…
A:
Q: Consider the titration of 25.00 mL of 0.03555 M Co2+ by 0.02784 M EDTA at pH 10.00. Kf is 1045.…
A: The amount of EDTA required to reach the endpoint of the titration is determined as shown below.…
Q: The formation constant (Kf) for calcium reacting with EDTA is 6.2 x108. A calcium-EDTA titration was…
A: Ca2+ + (EDTA)4- ⇔(Ca-EDTA)2- ; Kf =6.2 * 108 αy4-= [EDTA4-][EDTA] = [EDTA4-] = αy4-[EDTA] => Kf…
Q: A commercial lab received a batch of industrial wastewater samples for analysis. John plans to test…
A: The chemical equation for the reaction between EDTA and Ca2+ is: Ca2+ + EDTA ---->…
Q: 0.45 gm of calcium carbonate is dissolved in 1 liter of distilled water. 50 ml of this standard…
A: 1mL of standard hard water contains 1mg of CaCO3.
Q: Question attached
A: EDTA is ethylene diamine tetra acetic acid. It is a hexadentate ligand. The ability of multidentate…
Q: A 25.00 mL sample of water, buffered at a pH of 10, is titrated with 0.008989 M EDTA using the…
A: The hardness in water can be calculated using EDTA which binds with metal ions like Ca and Mg.
Q: Regarding EDTA titrations and other complex forming reactions, indicate T or F 1) The relevant…
A: 1) False 2) False (6 Binding sites) 3) True ( Eriochrome Black T changes color from blue to pink.)…
Q: Using the graph given, which metal can be titrated better with EDTA? A)Fe3+ B) Fe2+ C) Ca2+ D) Hg2+
A: From the graph ,it is clear that Fe 3+ has highest complex formation constant .
Q: why Mg (II) must be added when EDTA is standardized using a calcium standard solution and Eriochrome…
A:
Q: Which of the following is completely true regarding the titration of iron oxalate salt with EDTA? It…
A: Titration s also known as volumetric analysis which is a laboratory method for quantitative…
Q: 2. 25.00-mL aliquots of the solution in problem 1 are titrated with EDTA to the calmagite end point.…
A: (A) The volume of EDTA required for blank titration is 2.12 mL. The volume of EDTA required for…
Q: When using EDTA to determine metal ion concentration in a solution, what is the best way to overcome…
A: Masking agent is used to protect some component of the analyte from the reaction with EDTA and thus…
Q: QUESTION 1. Explain why Mg-EDTA complex is added to the titration mixture in the determination of…
A:
Q: Considering the graph given, what is the most suitable pH for the titration of calcium with EDTA?…
A: The determination of the calcium by titration with EDTA is one of the classic methods. The structure…
Q: 3. A 100 mL sample of hard water is titrated with 22.4 mL of the EDTA solution from problem 2. The…
A: Its given as
Q: * EDTA is used for titration of -5
A: EDTA stands for ethylenediamine tetra acetic acid. It can acts as ligand i.e electron pair donar.…
Q: Titration of 50.00 mL of 0.04715 M Na2C2O4 required 39.25 mL of a potassium permanganate solution.…
A: Given, Volume of Na2C2O4 = 50.00 mL Concentration of Na2C2O4 = 0.04715 M…
Q: An auxiliary competing agent is used in EDTA titrations to slow down the titration. keep some…
A: The solution is given below -
Q: How do I find Moles of EDTA required for a complexometric titration of Magnesium if I have the…
A: Molarity is defined as “the number of moles of solute present in per litre of solution”. It is…
Q: Why polydentate ligands are useful titrants compared to monodentate ligands in titration for metal…
A: To answer questions regarding complexometric titrations:
Q: The answer to the previous question I calculated was 0.0324 M of the standard Ca solution and now…
A: Complexometric titrations are those in which there is a ligand and a metal ions being titrated and…
Q: Which of the following causes the sharpest change in the equivalence point of a complexometric…
A: The question is based on the concept of complexometric titration. we have to identify the reason…
Q: To standardize EDTA solution, three independent titrations were conducted, each with 20 mL of 0.01…
A: “Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: EDTA cannot be used as a primary standard always. When can EDTA be used as a primary standard ?…
A: The acid-base titration is involves the reaction of acid with base to calculate the concentration of…
Q: An EDTA solution was prepared by dissolving approximately 4.0 g of disodium salt in approximately 1…
A: The concentration terms explains the mass of solute present in the total volume of the solution.…
Q: Compare the 2 quantities based on the given condition. Choose A If quantity in Column 1 is greater B…
A: Cu2+ is titrated with EDTA solution . This is a complexpmetric Titration.
Q: um in physiologic fluids can be determined by a complexometric titration with EDTA. In one such…
A: The method is used for the titration calcium with EDTA is called a complex metric titration. This…
Q: A 50.0ml sample of water containing both Ca+2 and Mg+2 is titrated with 16.54ml of 0.01104M EDTA in…
A: Calculation of total mol of EDTA used: nEDTA=Molarity×Volume=0.01104 mol/L×16.54×10-3 L=1.826×10-4…
Considering the graph given, what is the most suitable pH for the titration of calcium with EDTA?
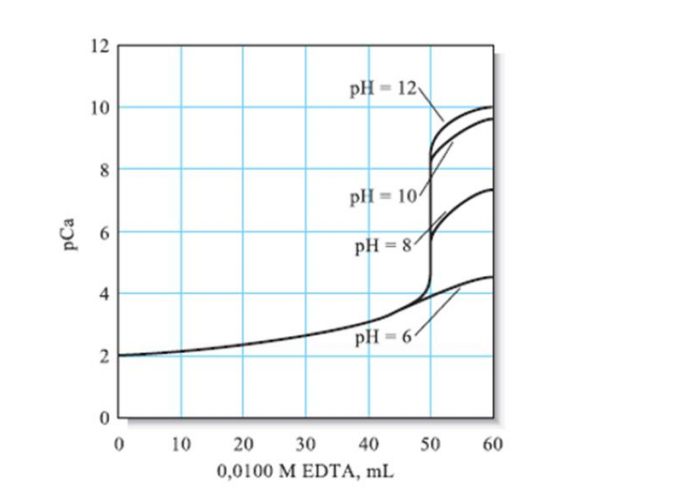
Step by step
Solved in 2 steps

- Please answer these questions about the spectrophotometric Analysis of commercial aspirinexplain the results of this experimet Table 2 Absorbance vs CoCl2 concentration Test Tube Number Cobalt Chloride Concentration (mol/mL) Absorbance at 510 nm 1 0.000 0.000 2 0.009 0.024 3 0.018 0.055 4 0.027 0.085 5 6 0.036 0.045 0.112 0.138 7 (Unknown) 0.088A series of standards were analyzed which gave the following results SOLUTION % Transmittance 0 mL of 0.100 mg/mL Hg + 2 mL tinchloride in a total volume of 75 mL 100 1.0 mL of 0.100 mg/mL Hg + 2 mL of tinchloride in a total volume of 75 mL 81.3 2.0 mL of 0.100 mg/mL Hg + 2 mL of tinchloride in a total volume of 75 mL 63.9 3.0 mL of 0.100 mg/mL Hg + 2 mL of tinchloride in a total volume of 75 mL 55 5.0 mL of 0.100 mg/mL Hg + 2 mL of tinchloride in a total volume of 75 mL 35.1 How would you make a Beer-Lambert Plot with this information?
- Use the calibration curve or linear regression equation to determine the concentration of FD&C blue dye No. 1 in Kool-aid. Food Coloring is 0.026 M in FD&C blue dye No. 1 7.5 x 10 -5 M is 29 mL -> 1000 mLThe Ksp value of magnesium fluoride is 5.16 X 10^-11 at 25 Celcius. MgF2 (s ) -> Mg^2+ (aq) +2F^- (aq)Using this formula Cu = Au x Cstd / Astd Where: C = concentration A = absorbance U = unknown/sample STD = standard Compute for the glucose concentration of the patient’s sample. (Note: The standard reading in the video is quite very high, so I am going to give you a value for the standard which you can use for your computation) Given: Absorbance of the standard = 0.075 Concentration of the standard is mentioned in the video. = 10.54 mmol/L Absorbance of unknown is in the video. = 0.083 Convert your answer to mmol/L or S.I. using the conversion factor for glucose which is 0.055
- Solution. Vol. of A solution (mL) [methyl red], M Absorbance 1 10.00 _1.64 x10^-16_ _0.256_ 2 15.00 _2.45 x10^-16_. _0.373_ 3 20.00 _3.27 x10^-16_. _0.486_ 4 25.00 _4.09 x10^-16_. _0.620_ Plot a graph of the absorbance (y axis) as a function of the concentration of methyl red (x axis). What is the slope of the graph?Boxes 1-2. box 1 answer choices: 6, 3, 5, 4, 1, or 2. box 2 answer choices: -0.90 V, +0.90 V, +2.42 V, +1.04 V, -2.42 V, or -1.04 V.The students conducted the assay for LDH activity using the two serum samples. Each cuvette (path length 1 cm) contained 3ml of a suitable assay buffer (including pyruvate as substrate). 20 microlitres of either serum was added to the cuvette and the absorbance values immediately recorded at the optimum wavelength for a period of 5 minutes (absorbance readings taken every 30 seconds). Protein concentration of serum sample (mg/ml) Change in absorbance at optimum wavelength per minute Control serum (C) 8 -0.04 Diseased serum (D) 7.8 -0.6 1c. Using the molar absorption coefficient of NADH as 6220 M-1 cm-1, and by application of the Beer-Lambert law, estimate the enzyme activity in the two samples (C and D). Express activity as moles per second. 1d. Estimate the specific activity of the two samples (moles per second per microgram).
- A standard of solution was put through appropriate dilutions to give the concentrations of glucose (mM) shown in the accompanying table. The absorbances in the table (1.00-cm cells) were recorded at 490 m table. Use the method of least squares to find an equation relating absorbance and the concentration of glucose.Please help me answer number 1 and 2 and please highlight the answerWhat are the requirements of a primary standard?







