carbanions-ions in which a carbon atom has three bonds and a lone pair of electrons and bears a negative charge. Draw another contribut- ing structure for the allyl anion. Now using cartoon representations, draw the three orbit- als that represent the delocalized 7 system (look at Figure 1.26 for a hint). Which of the three orbitals are populated with electrons? H H. H Allyl anion
carbanions-ions in which a carbon atom has three bonds and a lone pair of electrons and bears a negative charge. Draw another contribut- ing structure for the allyl anion. Now using cartoon representations, draw the three orbit- als that represent the delocalized 7 system (look at Figure 1.26 for a hint). Which of the three orbitals are populated with electrons? H H. H Allyl anion
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 16CTQ: Now consider the fully formed molecule on the right side of Figure 3.7. a. Draw a Lewis structure of...
Related questions
Question
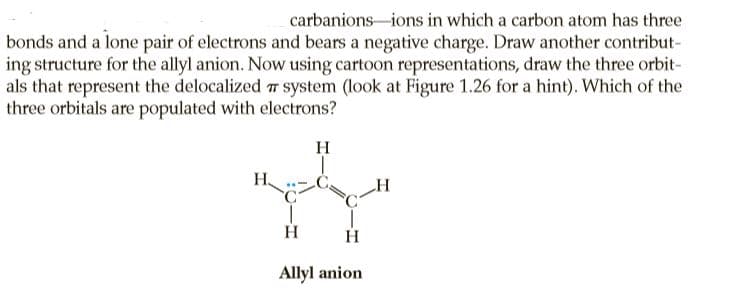
Transcribed Image Text:carbanions-ions in which a carbon atom has three
bonds and a lone pair of electrons and bears a negative charge. Draw another contribut-
ing structure for the allyl anion. Now using cartoon representations, draw the three orbit-
als that represent the delocalized 7 system (look at Figure 1.26 for a hint). Which of the
three orbitals are populated with electrons?
H
H.
H
Allyl anion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning