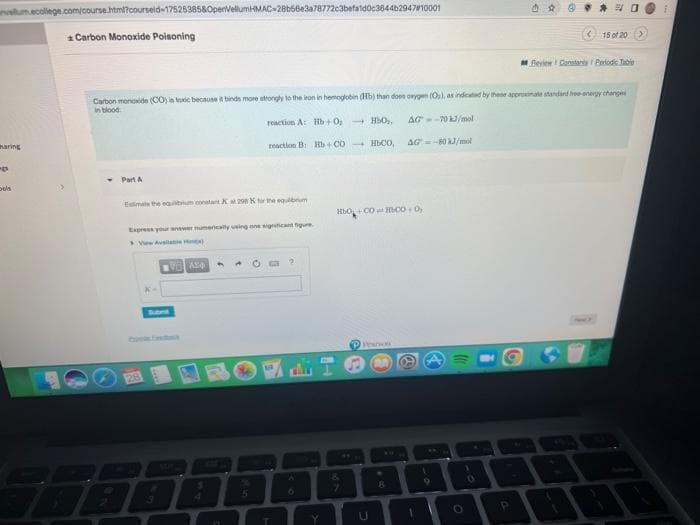
Carbon monoxide (CO) is toxic because it binds more strongly to the iron in hemoglobin (Hb) than does oxygen (Os), as indicated by these approximate standard free-energy changes in blood reaction A: Hb+0,0, AG-70 kJ/mol reaction B Bb+COCO, AG--80 kJ/mol Part A Estimate the earum constant Kat 298 K to the equlbum Express your answer numencally using one significant figure KA Aze 4 ? Hbo Coco+0,
Carbon monoxide (CO) is toxic because it binds more strongly to the iron in hemoglobin (Hb) than does oxygen (Os), as indicated by these approximate standard free-energy changes in blood reaction A: Hb+0,0, AG-70 kJ/mol reaction B Bb+COCO, AG--80 kJ/mol Part A Estimate the earum constant Kat 298 K to the equlbum Express your answer numencally using one significant figure KA Aze 4 ? Hbo Coco+0,
Chapter6: Organic Chemistry
Section: Chapter Questions
Problem 66E
Related questions
Question
Transcribed Image Text:velum.ecollege.com/course.html?courseld-17525385&OpenVellumHMAC-28b56e3a78772c3befa1d0c3644b2947#10001
haring
D
Dels
Carbon Monoxide Poisoning
55
Part A
Carbon monoxide (CO) is toxic because it binds more strongly to the iron in hemoglobin (Hb) than does oxygen (Os), as indicated by these approximate standard free-energy changes
in blood
reaction A: Hb+O,
HBO₂,
AG-70 kJ/mol
reaction B: Hb+CO HbC0, AG-80 kJ/mol
Estimate the equum constant Kat 298 K for the equum
Express your answer mumencally using one significant figure
Vw Availa
K-
VE AS
4
5
T
?
6
Y
Hb+0000+0₂
7
Pleas
8
1
9
O
0
리ㅁ
P
<15 of 20
Review Constants / Periodic Table
¡
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning