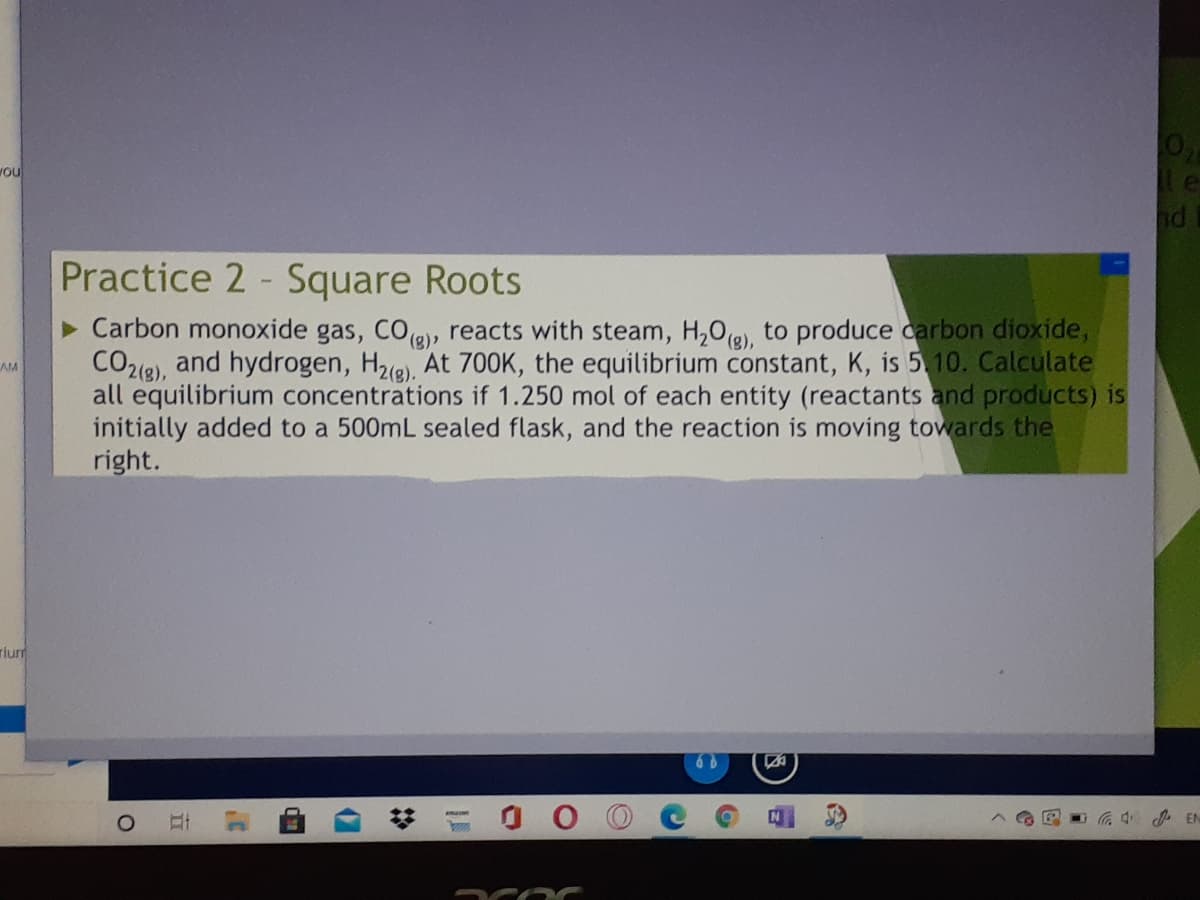
Carbon monoxide gas, COg), reacts with steam, H20(8), to produce carbon dioxide, CO2(e), and hydrogen, H2e), At 700K, the equilibrium constant, K, is 5 10. Calculate all equilibrium concentrations if 1.250 mol of each entity (reactants and products) is initially added to a 500mL sealed flask, and the reaction is moving towards the right.
Carbon monoxide gas, COg), reacts with steam, H20(8), to produce carbon dioxide, CO2(e), and hydrogen, H2e), At 700K, the equilibrium constant, K, is 5 10. Calculate all equilibrium concentrations if 1.250 mol of each entity (reactants and products) is initially added to a 500mL sealed flask, and the reaction is moving towards the right.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.81P
Related questions
Question
Please answer question and just send me the paper solutions ASAP and dont give me the wrong solution
Transcribed Image Text:0,
nd
Practice 2 - Square Roots
> Carbon monoxide gas, COe, reacts with steam, H,0(e), to produce carbon dioxide,
CO26), and hydrogen, Hze), At 700K, the equilibrium constant, K, is 5 10. Calculate
all equilibrium concentrations if 1.250 mol of each entity (reactants and products) is
initially added to a 500mL sealed flask, and the reaction is moving towards the
right.
(),
AM
Tiurm
I EN
%2:
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
