Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU2: Smells: Molecular Structure And Properties
SectionU2.3: Honc If You Like Molecules: Bonding Tendencies
Problem 1E
Related questions
Question
Use the following table to rank the compounds in order of increasing (weakest to strongest) lattice energy.
*Picture attached*
Answer Options:
A. MT2 < MQ2 < MR
B. MR < MQ2 < MT2
C. MR < MT2 < MQ2
D. MQ2 < MR < MT2
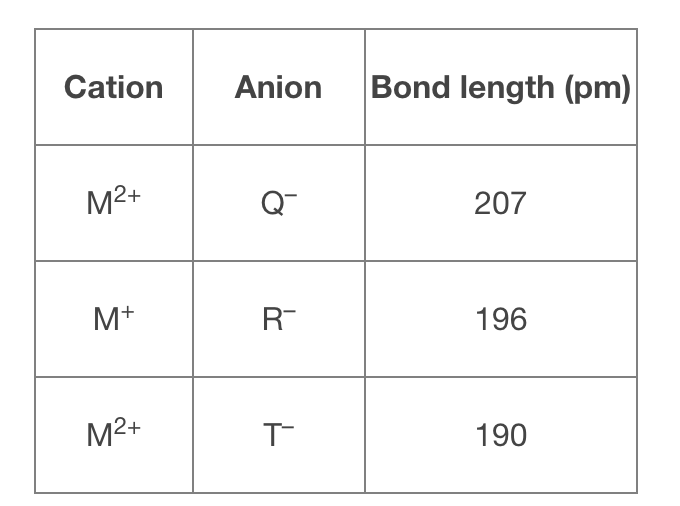
Transcribed Image Text:Cation
Anion
Bond length (pm)
M2+
Q-
207
M+
R-
196
M²+
190
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning