Cd2+/Cd = -0.403 V. To protect Cadmium from corrosion through sacrificial anodic protection, which of the following metals will be most suitable:
Cd2+/Cd = -0.403 V. To protect Cadmium from corrosion through sacrificial anodic protection, which of the following metals will be most suitable:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 62P
Related questions
Question
Do both in 2 min. I will give u like
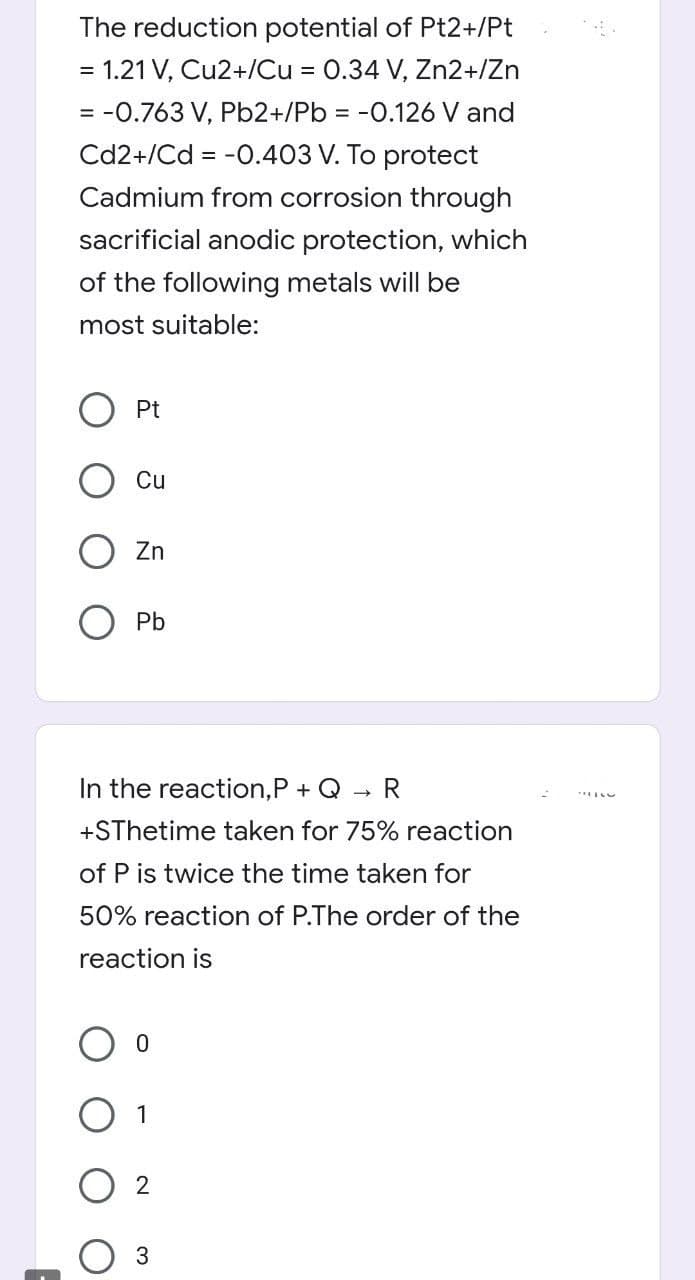
Transcribed Image Text:The reduction potential of Pt2+/Pt
= 1.21 V, Cu2+/Cu = 0.34 V, Zn2+/Zn
= -0.763 V, Pb2+/Pb = -0.126 V and
Cd2+/Cd = -0.403 V. To protect
Cadmium from corrosion through
sacrificial anodic protection, which
of the following metals will be
most suitable:
Pt
Cu
Zn
O Pb
In the reaction,P + Q
R
+SThetime taken for 75% reaction
of P is twice the time taken for
50% reaction of P.The order of the
reaction is
O 1
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning