CH3 O CH3 OC H3 HO-C-CH2CH2CH3 CH3CH2-0-CHCH3 CH3-C-CH2CH2CH3 H-C-C-CH2CH3 H-C-C-CH3 H. CH3 A B E has the lowest boiling point the least soluble in water Answer: Answer: a non-enolizable carbonyl compound results in bubbling upon addition of 10% NaHCO3 Answer: Answer: reacts with 2,4-DNP but not with Tollens' gives an iodoform upon reaction with I2/KI in NaOH reagent Answer: Answer:
CH3 O CH3 OC H3 HO-C-CH2CH2CH3 CH3CH2-0-CHCH3 CH3-C-CH2CH2CH3 H-C-C-CH2CH3 H-C-C-CH3 H. CH3 A B E has the lowest boiling point the least soluble in water Answer: Answer: a non-enolizable carbonyl compound results in bubbling upon addition of 10% NaHCO3 Answer: Answer: reacts with 2,4-DNP but not with Tollens' gives an iodoform upon reaction with I2/KI in NaOH reagent Answer: Answer:
Chapter17: Alcohols And Phenols
Section17.2: Properties Of Alcohols And Phenols
Problem 3P: The following data for isomeric four-carbon alcohols show that there is a decrease in boiling point...
Related questions
Question
Consider the following compounds of comparable molecular weight
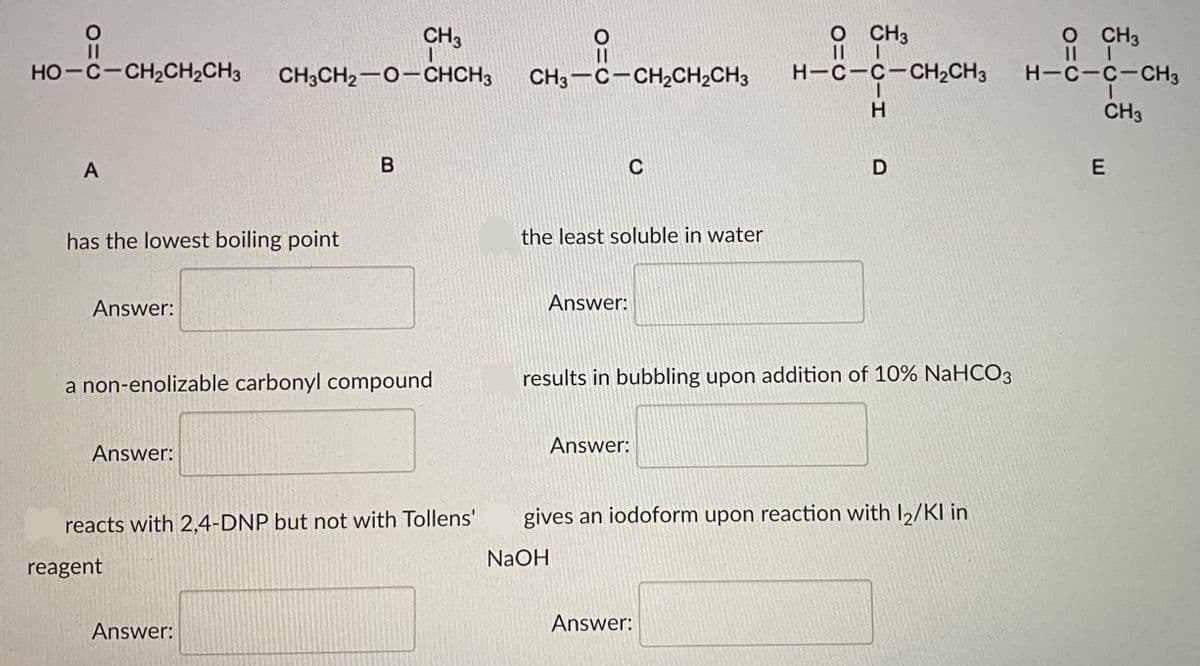
Transcribed Image Text:CH3
O CH3
O CH3
HO-C-CH2CH2CH3
CH3CH2-0-CHCH3
CH3-C-CH,CH2CH3
H-C-C-CH2CH3
H-C-C-CH3
H.
CH3
A
C
has the lowest boiling point
the least soluble in water
Answer:
Answer:
a non-enolizable carbonyl compound
results in bubbling upon addition of 10% NaHCO3
Answer:
Answer:
reacts with 2,4-DNP but not with Tollens'
gives an iodoform upon reaction with I2/KI in
NaOH
reagent
Answer:
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning