CH3-C-NH, CH3-C-NH2 CH3-C=NH2 Largest contributor (#1) Minor contributor (#3) Major contributor (#2) In summary, resonance form C is the best representation of this molecule. However, consid- eration of the other contributing structures, even the minor contributor, helps us to identity positions that are electron-rich (8-) or electron-deficient (8+). 8- - Electron-rich site CH3-C-NH2 8+ 8+ Electron-deficient sites :kill 2.26 For each of the following, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. NH (b) (a) (c) H. CH3-C-CEN (e) (d) (f) G. 2.27 In the compounds shown below, the six-membered rings are called benzene rings. Such rings are commonly found in natural products, and we will learn more about the remarkable stahility of these ring systems in Chapter 17. Because of resonance effects with the attache
CH3-C-NH, CH3-C-NH2 CH3-C=NH2 Largest contributor (#1) Minor contributor (#3) Major contributor (#2) In summary, resonance form C is the best representation of this molecule. However, consid- eration of the other contributing structures, even the minor contributor, helps us to identity positions that are electron-rich (8-) or electron-deficient (8+). 8- - Electron-rich site CH3-C-NH2 8+ 8+ Electron-deficient sites :kill 2.26 For each of the following, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. NH (b) (a) (c) H. CH3-C-CEN (e) (d) (f) G. 2.27 In the compounds shown below, the six-membered rings are called benzene rings. Such rings are commonly found in natural products, and we will learn more about the remarkable stahility of these ring systems in Chapter 17. Because of resonance effects with the attache
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.SE: Something Extra
Problem 56GP: Long-range coupling between protons more than two carbon atoms apart is sometimes observed when ...
Related questions
Question
Hello, I need help with question 26 a-f. I need help with the while problem.
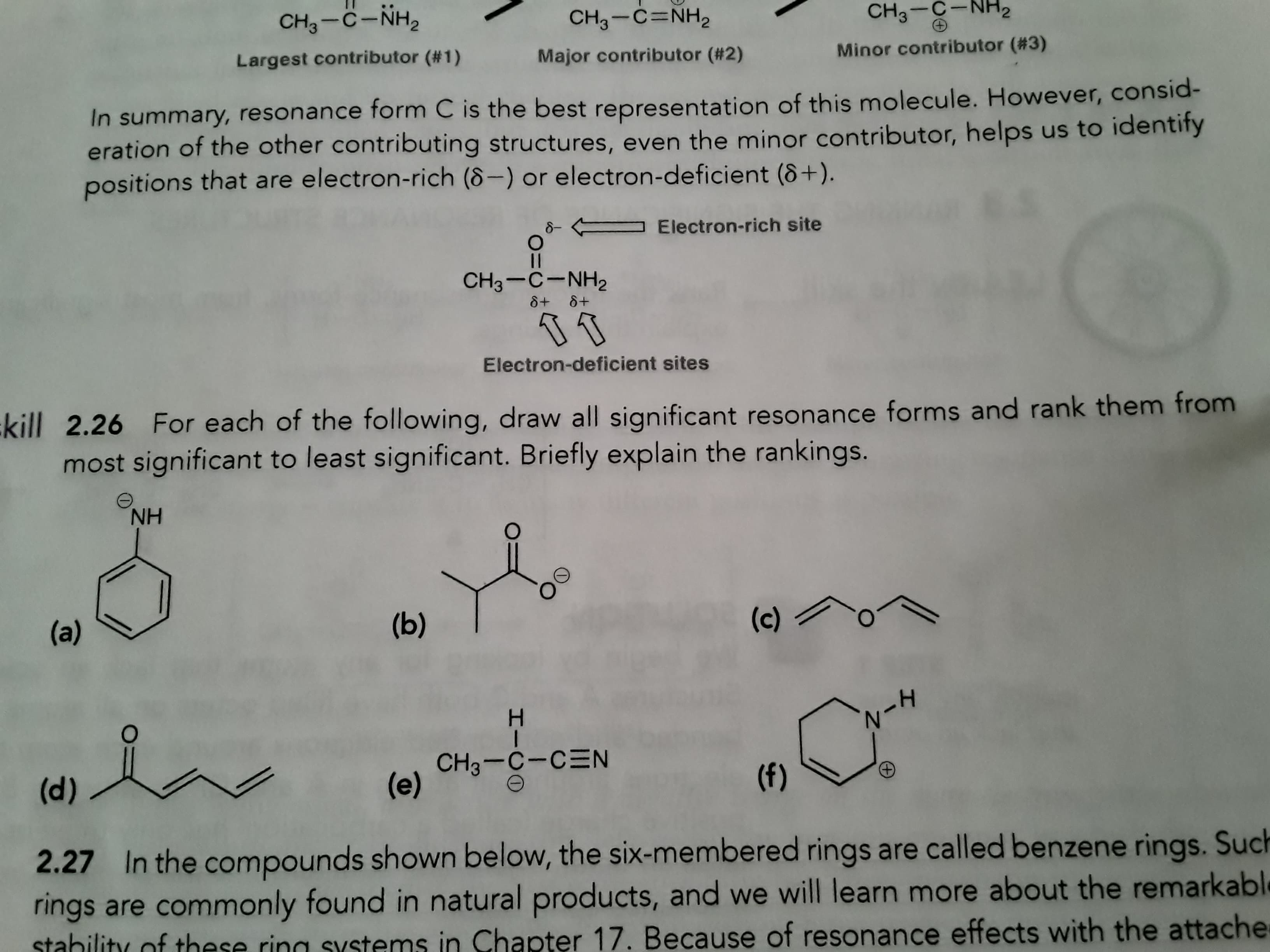
Transcribed Image Text:CH3-C-NH,
CH3-C-NH2
CH3-C=NH2
Largest contributor (#1)
Minor contributor (#3)
Major contributor (#2)
In summary, resonance form C is the best representation of this molecule. However, consid-
eration of the other contributing structures, even the minor contributor, helps us to identity
positions that are electron-rich (8-) or electron-deficient (8+).
8-
- Electron-rich site
CH3-C-NH2
8+ 8+
Electron-deficient sites
:kill 2.26 For each of the following, draw all significant resonance forms and rank them from
most significant to least significant. Briefly explain the rankings.
NH
(b)
(a)
(c)
H.
CH3-C-CEN
(e)
(d)
(f)
G.
2.27 In the compounds shown below, the six-membered rings are called benzene rings. Such
rings are commonly found in natural products, and we will learn more about the remarkable
stahility of these ring systems in Chapter 17. Because of resonance effects with the attache
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning