structures are fragments of major product of dehydration reaction of 2-methylbutan-2-ol. need to know why some of them have base peaks of 97. B and D have base peaks of 97.1, and A and C have peaks of 57.1
structures are fragments of major product of dehydration reaction of 2-methylbutan-2-ol. need to know why some of them have base peaks of 97. B and D have base peaks of 97.1, and A and C have peaks of 57.1
Chapter18: Ethers And Epoxides; Thiols And Sulfides
Section18.SE: Something Extra
Problem 46AP
Related questions
Question
structures are fragments of major product of dehydration reaction of 2-methylbutan-2-ol. need to know why some of them have base peaks of 97. B and D have base peaks of 97.1, and A and C have peaks of 57.1

Transcribed Image Text:Based on the structures of Compounds A-D, explain why some of these species have very
intense m/z=97 peaks and others do not (you will need to draw structures).
Consider compounds A -D. Which of these compounds could be considered Zaitsev products,
and which are Hofmann products?
Is the observed product distribution consistent with Zaitsev's rule?
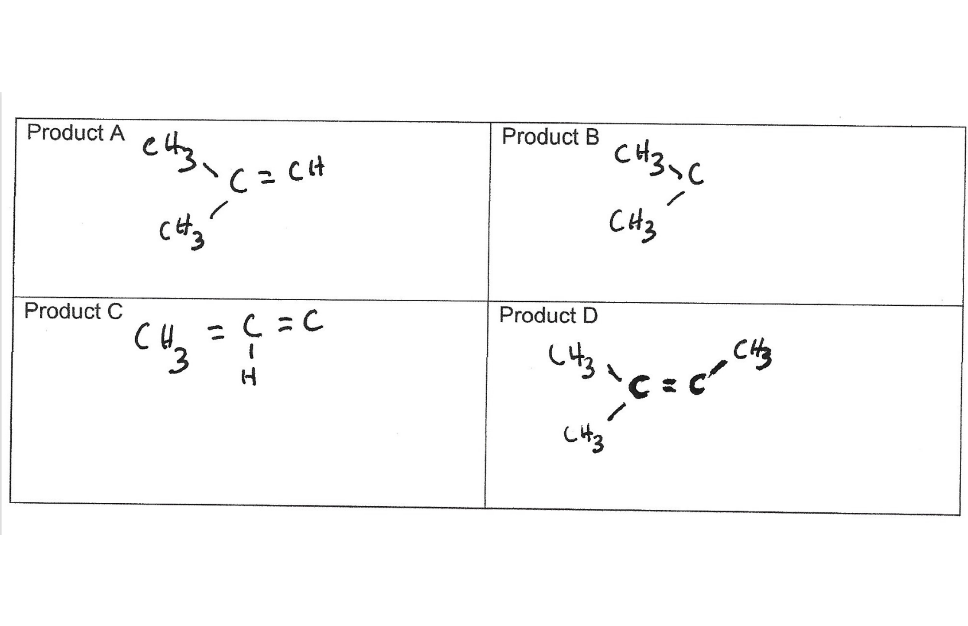
Transcribed Image Text:Product A
Product B
CHz
Product C
Product D
CH. = C=C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax