Chalcopyrite (CuFeS2), is an important source of copper. A typical chalcopyrite ore contains about 0.75% Cu. What volume of sulfur dioxide at 25°C and 1 atm pressure is produced when one boxcar load ( 4 x 10 ft³) of chalcopyrite ore (density = 2.6 -9 is roasted? cm3 Assume all the sulfur in the ore is converted to SO2 and no other source of sulfur is present. Find the following: (do not express your answer in scientific notation, do not input the unit space or comma, your answer should be in two decimal places)
Chalcopyrite (CuFeS2), is an important source of copper. A typical chalcopyrite ore contains about 0.75% Cu. What volume of sulfur dioxide at 25°C and 1 atm pressure is produced when one boxcar load ( 4 x 10 ft³) of chalcopyrite ore (density = 2.6 -9 is roasted? cm3 Assume all the sulfur in the ore is converted to SO2 and no other source of sulfur is present. Find the following: (do not express your answer in scientific notation, do not input the unit space or comma, your answer should be in two decimal places)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 48QAP
Related questions
Question

Transcribed Image Text:Chalcopyrite (CuFeS2), is an important source of copper. A typical
chalcopyrite ore contains about
0.75% Cu. What volume of sulfur dioxide at 25°C and 1 atm pressure is
produced when one boxcar
g.
load (4 x 10 ft³) of chalcopyrite ore (density = 2.6 ) is roasted?
cm3
Assume all the sulfur in
the ore is converted to SO2 and no other source of sulfur is present.
Find the following: (do not express your answer in scientific notation, do not input the unit,
space or comma, your answer should be in two decimal places)
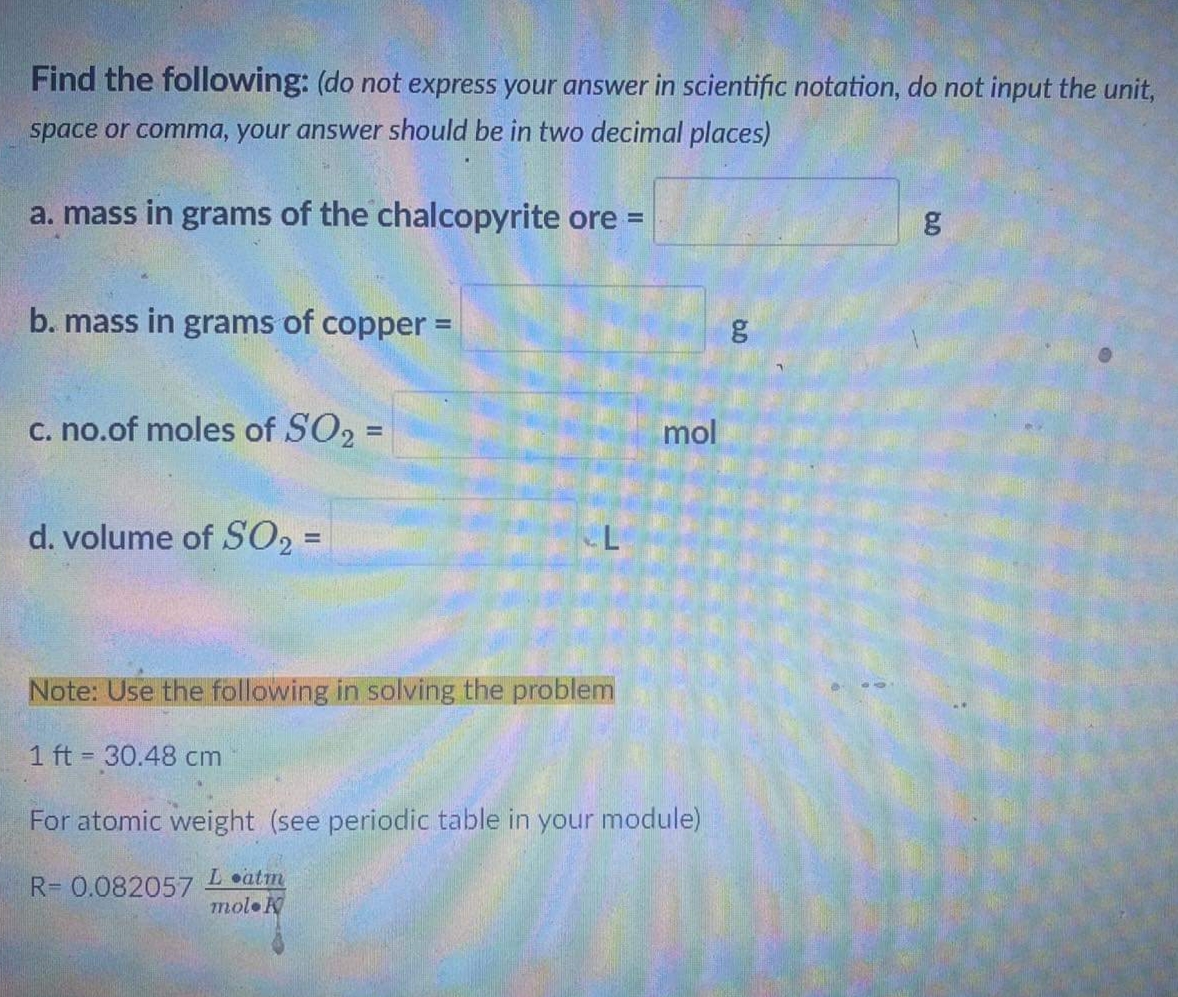
Transcribed Image Text:Find the following: (do not express your answer in scientific notation, do not input the unit,
space or comma, your answer should be in two decimal places)
a. mass in grams of the chalcopyrite ore =
b. mass in grams of copper =
c. no.of moles of SO, =
mol
d. volume of SO2 =
Note: Use the following in solving the problem
1 ft 30.48 cm
For atomic weight (see periodic table in your module)
Latm
R= 0.082057
molo N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning