Chapter 15 HW Exercise 15.77 - Enhanced - with Feedback and Hints artA What MISSED THIS? Read Section 15.6 (Pages 656 - 661); Watch KCV 15.6, IWE 15.9. your Consider this three-step mechanism for a reaction: Part A Ch(g) )= 2C1(g) (fast) What is the overall reaction? Figure Cl(g)+ CHC3 (g) Express your answer as a chemical eqe - HCl(g) + CCI (g) (slow). ks CCL (g) > View Available Hint(s) Cl(g) + CCls (g) (fast) AE DA chemical reaction does not occur for this
Chapter 15 HW Exercise 15.77 - Enhanced - with Feedback and Hints artA What MISSED THIS? Read Section 15.6 (Pages 656 - 661); Watch KCV 15.6, IWE 15.9. your Consider this three-step mechanism for a reaction: Part A Ch(g) )= 2C1(g) (fast) What is the overall reaction? Figure Cl(g)+ CHC3 (g) Express your answer as a chemical eqe - HCl(g) + CCI (g) (slow). ks CCL (g) > View Available Hint(s) Cl(g) + CCls (g) (fast) AE DA chemical reaction does not occur for this
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 71QAP: For a certain reaction, Ea is 135 kJ and H=45 kJ. In the presence of a catalyst, the activation...
Related questions
Question
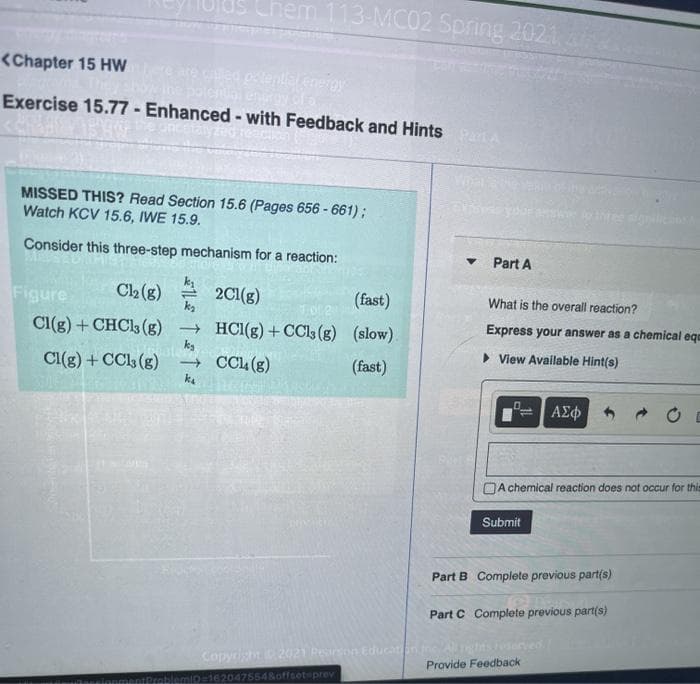
Transcribed Image Text:Chem 113-MC02 Spring 2021
<Chapter 15 Hw
ed potential erergy
Exercise 15.77 - Enhanced - with Feedback and Hints PartA
What
MISSED THIS? Read Section 15.6 (Pages 656 - 661);
Watch KCV 15.6, IWE 15.9.
hree ala
Consider this three-step mechanism for a reaction:
Part A
C2 (g)
2Cl(g)
ka
(fast)
Figure
What is the overall reaction?
Express your answer as a chemical ege
Cl(g) + CHCl3 (g)
→ HCI(g) + CCls (g) (slow).
kg
> View Available Hint(s)
Cl(g) + CC (g)
k.
→ CCL(g)
(fast)
ΑΣφ
OA chemical reaction does not occur for this
Submit
Part B Complete previous partís)
Part C Complete previous part(s)
Copyright 2021 Person EducanAgns ved
Provide Feedback
ProblemiD=16204755480ffeet-prev
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning