Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
What would the partial pressure of water be at 21.3 degrees Celsius from the chart below?
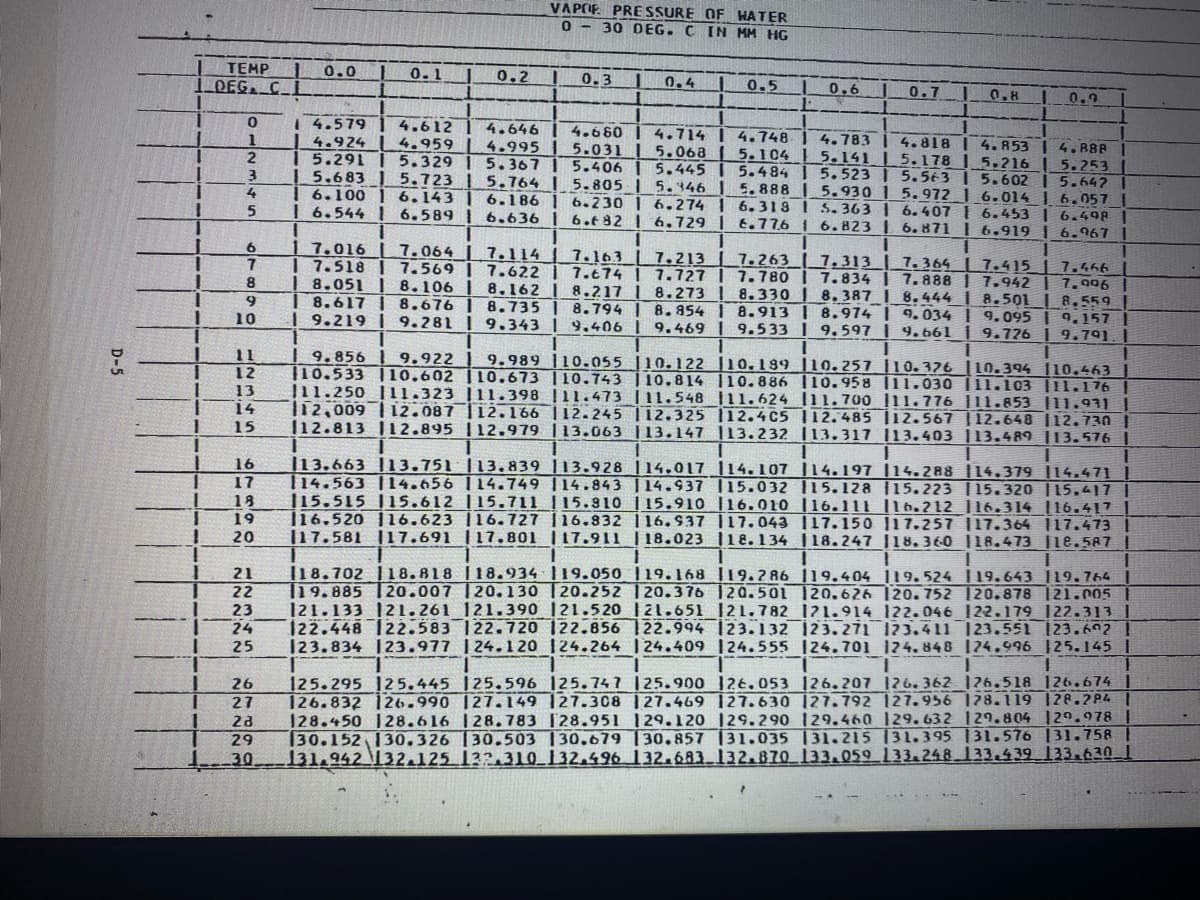
Transcribed Image Text:VAPCIF PRE SSURE OF HATER
0 - 30 DEG. C IN MM HG
TEMP
LDEG. C.
0.0
0.1
0,2
0.3
0.4
0.5
| 0.6
0.7
0.9
1 4.579
4.924 | 4.959 | 4.995 | 5.031 5.068 1 5.104 5.141 | 5.178 5.216 | 5.253
I 5.291 | 5.329 | 5.367 5.406 5.445 | 5.484 I 5.523 | 5.563 | 5.602 i 5.642
| 5.683 5.723 | 5.764 | 5.805 I 5.146 | 5.888 5.930 | 5.972 | 6.014 | 6,057_
1 6.100 | 6.143 | 6.186 | 6.230 | 6.274 I 6.313I 5.363 | 6.407 | 6.453 | 6.498
| 6.544 | 6.589
4.612
4.646
4.660
4.714 | 4.748 I 4.783 | 4.818 | 4.853 | 4. 888
1
3.
4
6.636 | 6.t 82 | 6.729 | 6.77,6 1 6.823 | 6.871 | 6.919 | 6.967
17.016 7.064
7.518 I 7.569 | 7.622
| 8.051 | 8.106 | 8.162 | 8.217 | 8.273 8.330 | 8.387 I 8,444 | 8.501 | 8.559.
| 8.617 | 8.676 | 8.735 | 8.794 | 8.854 I 8.913 | 8.974 | 9.034 | 9.095 | 9.157||
| 9.219 | 9.281 | 9.343 | 9.406 | 9.469 | 9.533 | 9.597 | 9.661
7.114 | 7.163 7.213 7.263 L 7.313 | 7. 364 | 7.415| 7.466
7.674 | 7.727 | 7.780 I 7.834 | 7.888 | 7.942
7.
8
7.906
9.
10
11
12
13
14
9.726 | 9.791. |
9.856 9.922 | 9.989 |10.055 |10.122 110.199 |10.257 |10.376 |10.394 110.463 |
T10.533 |10.602 |10.673 |10.743 | 10.814 110.886 |10.958 111.030 |11.103 |11.176 I
|11.250 |11.323 |11.398 |11.473 |11.548 11.624 I11.700 |11.776 |11.853 |11.931 I
li2,009 | 12.087 |12.166 |12.245 12.325 112.4C5 I12.485 12.567 |12.648 |12.730 I
|12.813 |12.895 |12.979 |13.063 |13.147 113.232 |13.317 |13.403 |13.489 |13.576 I
15
|13.663 |13.751 |13.839 |13.928 |14.017 |14.107 |14.197 |14.288 |14.379 |14.471 I
114.563 |14.656 |14.749 |14.843 14.937 15.032 I15.128 15.223 |15.320 |15.417 I
|15.515 |15.612 |15.711 |15.310 | 15.910 |16.010 |16.111 |16.212 J16.314 |16.417 I
|16.520 |16.623 |16.727 |16.832 |16.937 l17.043 I17.150 |17.257 |17.364 117.473 i
|17.581 |17.691 |17.801 |17.911 |18.023 |18.134 |18.247 118.360 |18.473 |1e.587 |
16
17
18
19
20
1.
|18.702 |18.818 |18.934 |19.050 19.168 19.286 119.404 119, 524 |19.643 |19.764I
T19.885 120.007 |20.130 20.252 120.376 120.501 120.626 120. 752 120.878 121.005 |
121.133 121.261 121.390 121.520 121.651 121.782 1?1.914 122.046 122.179 122.313 |
122.448 122.583 122.720 122.856 122.994 123.132 123.271 123.411 |23.55i [23.602
123.834 123.977 |24. 120 124.264 |24.409 124.555 124.701 124. 848 124.996 |25.145 |
21
22
23
24
25
1.
125.295 125.445 125.596 125.747 125.900 126.053 126.207 126.362126.518 126.674
126.832 126.990 127.149 127.3C8 127.469 127.630 127.792 127.956 178.119 128.2P4
128.450 128.616 |28.783 128.951 |29.120 129.290 129.460 129. 632 129.804 129.978
130.152 130.326 30.503 130.679 (30.857 131.035 131.215 131.395 131.576 131.758 |
131.942 132.125 132.310 132.496 132.683 132.870 133.059 133.248 133.439 133.630
26
27
28
29
30
D-5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
