The plot below shows the distribution of molecular velocities for two gases (A and B) at the same temperature. Which of the following statements about these gases is TRUE? Recall that a "particle" can be an atom or a molecule. A Molecular Velocity 1200 200 400 600 800 1000 A. The particles in gas A have a lower average kinetic energy (KEavg) than the particles in gas B B. Gas A has a higher molar mass than gas B C. Gas A diffuses/effuses more slowly than gas B Both B and C O All of A, B, and C O B only OC only OA only Fraction of Molecules B.
The plot below shows the distribution of molecular velocities for two gases (A and B) at the same temperature. Which of the following statements about these gases is TRUE? Recall that a "particle" can be an atom or a molecule. A Molecular Velocity 1200 200 400 600 800 1000 A. The particles in gas A have a lower average kinetic energy (KEavg) than the particles in gas B B. Gas A has a higher molar mass than gas B C. Gas A diffuses/effuses more slowly than gas B Both B and C O All of A, B, and C O B only OC only OA only Fraction of Molecules B.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 70QAP: Given that 1.00 mol of neon and 1.00 mol of hydrogen chloride gas are in separate containers at the...
Related questions
Question
Please explain why.
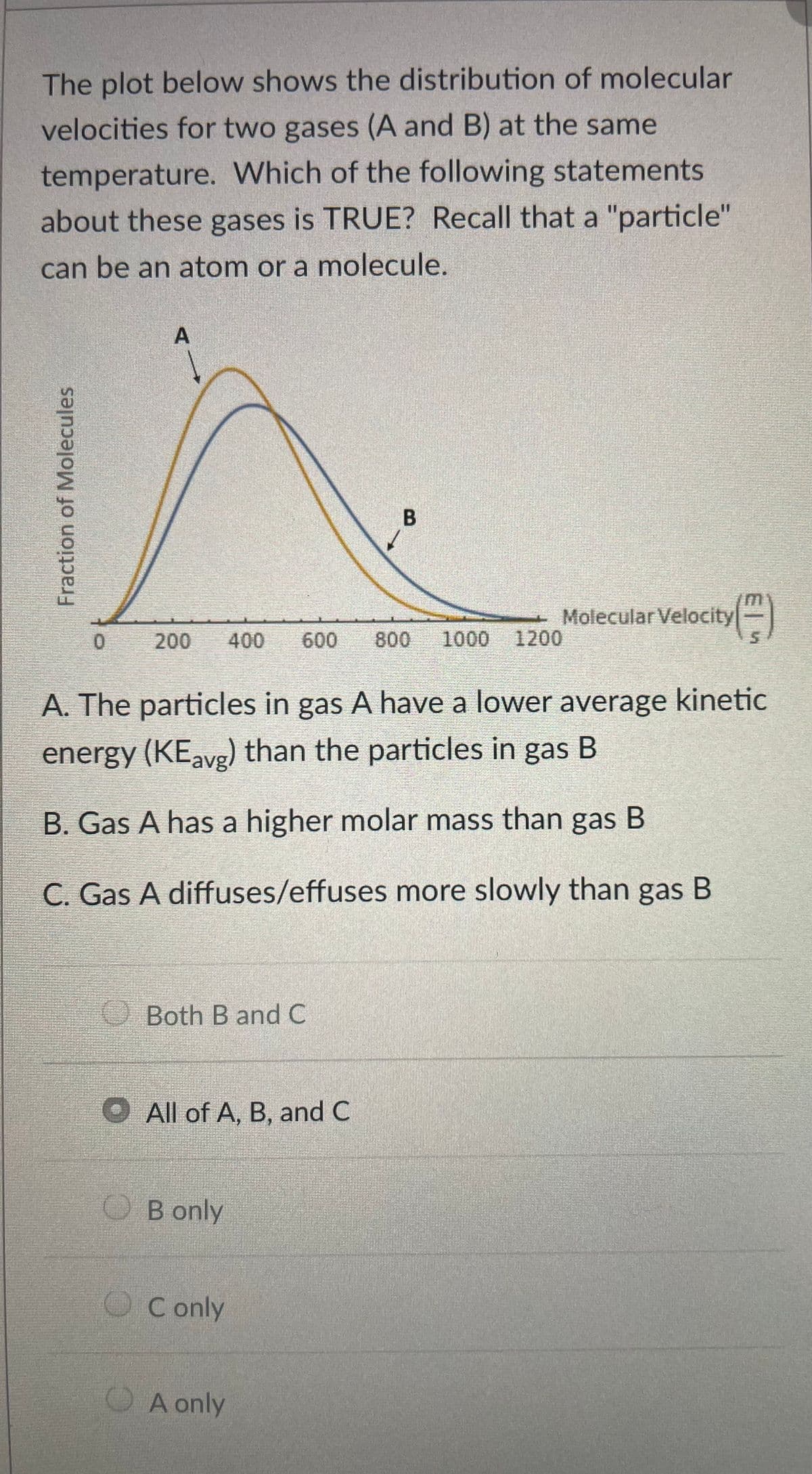
Transcribed Image Text:The plot below shows the distribution of molecular
velocities for two gases (A and B) at the same
temperature. Which of the following statements
about these gases is TRUE? Recall that a "particle"
can be an atom or a molecule.
()
Molecular Velocity
200
400
600
800
1000 1200
A. The particles in gas A have a lower average kinetic
energy (KEavg) than the particles in gas B
B. Gas A has a higher molar mass than gas B
C. Gas A diffuses/effuses more slowly than gas B
OBoth B and C
O All of A, B, and C
O B only
OC only
OA only
Fraction of Molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning