Check Your Understanding A. Direction: Determine the number of protons, neutrons and electrons in an atom/anion/cation. Complete the table below. Item number 1 is given as an example. Atom Symbol Mass Atomic Number Number Number & Number Number of of of Electron Neutron 74 Charge Proton Iodine Rubidium Neon 127 85 53 37 53 54 Rb Ne 20 10 O2. Oxygen Silver Phosphorus 16 8. Ag pa- 108 47 31 15
Check Your Understanding A. Direction: Determine the number of protons, neutrons and electrons in an atom/anion/cation. Complete the table below. Item number 1 is given as an example. Atom Symbol Mass Atomic Number Number Number & Number Number of of of Electron Neutron 74 Charge Proton Iodine Rubidium Neon 127 85 53 37 53 54 Rb Ne 20 10 O2. Oxygen Silver Phosphorus 16 8. Ag pa- 108 47 31 15
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 15P: In J. J. Thompson’s experiment depicted in Figures 1.10 and 1.11, assume that the electrons are...
Related questions
Question
100%
answer all
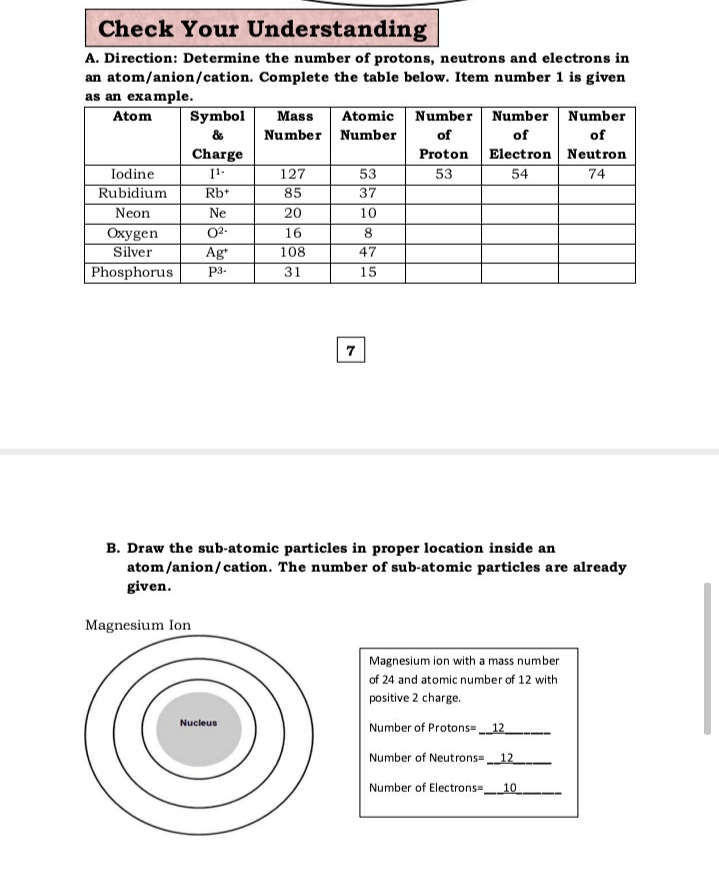
Transcribed Image Text:Check Your Understanding
A. Direction: Determine the number of protons, neutrons and electrons in
an atom/anion/cation. Complete the table below. Item number 1 is given
as an example.
Atom
Symbol
Mass
Atomic Number Number Number
&
Number Number
of
of
of
Charge
Electron Neutron
Proton
53
Iodine
Rubidium
127
53
54
74
Rb+
85
37
Neon
Ne
20
10
Охуgen
Silver
02-
16
8
Ag
Phosphorus
108
47
P3-
31
15
7
B. Draw the sub-atomic particles in proper location inside an
atom/anion/cation. The number of sub-atomic particles are already
given.
Magnesium Ion
Magnesium ion with a mass number
of 24 and atomic number of 12 with
positive 2 charge.
Nucleus
Number of Protons= 12
Number of Neutrons12
Number of Electrons-10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning