Check your Understanding Concept Map Directions: Complete the concept map .Choose your answer from the word list below. ELECTRON CONFIGURATION RULES 1 HUND'S RULE DEFINITION electrons will occupy the orbitals having lower energies before occupying higher energy orbitals 3 4 EXAMPLE 5 Lithium 1l 1 6 1s? 2s! Word List *Aufbau principle * Lithium | 1 *Lithium 1s? 2s' Pauli's exclusion principle *states that no two electrons can have equal values for all four quantum numbers. All the subshells in an orbital must be singly occupied before any subshell is doubly occupied
Check your Understanding Concept Map Directions: Complete the concept map .Choose your answer from the word list below. ELECTRON CONFIGURATION RULES 1 HUND'S RULE DEFINITION electrons will occupy the orbitals having lower energies before occupying higher energy orbitals 3 4 EXAMPLE 5 Lithium 1l 1 6 1s? 2s! Word List *Aufbau principle * Lithium | 1 *Lithium 1s? 2s' Pauli's exclusion principle *states that no two electrons can have equal values for all four quantum numbers. All the subshells in an orbital must be singly occupied before any subshell is doubly occupied
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section7.3: Electron Configuration Of Atoms
Problem 3CYU: Using the periodic table and without looking at Table 7.3, write electron configurations for the...
Related questions
Question
answer all
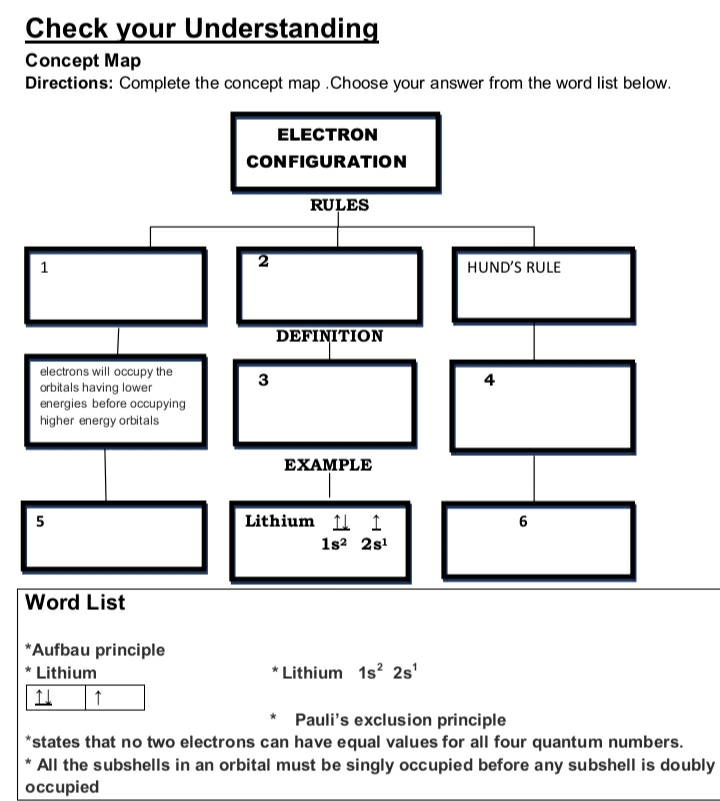
Transcribed Image Text:Check your Understanding
Concept Map
Directions: Complete the concept map .Choose your answer from the word list below.
ELECTRON
CONFIGURATION
RULES
1
HUND'S RULE
DEFINITION
electrons will occupy the
orbitals having lower
energies before occupying
higher energy orbitals
3
4
EXAMPLE
5
Lithium 1l 1
6
1s2 2s!
Word List
*Aufbau principle
* Lithium
| 11
* Lithium 1s? 2s'
Pauli's exclusion principle
*states that no two electrons can have equal values for all four quantum numbers.
* All the subshells in an orbital must be singly occupied before any subshell is doubly
occupied
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
