Part A What is the only possible value of mę for an electron in an s orbital? Express your answer numerically. • View Available Hint(s) Submit Part B What are the possible values of me for an electron in a d orbital? Express your answer numerically with sequential values separated by commas. • View Available Hint(s) Submit
Part A What is the only possible value of mę for an electron in an s orbital? Express your answer numerically. • View Available Hint(s) Submit Part B What are the possible values of me for an electron in a d orbital? Express your answer numerically with sequential values separated by commas. • View Available Hint(s) Submit
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 28P: Quantum mechanics predicts that the energy of the ground state of the H atom is 13.6eV . Insight...
Related questions
Question
Transcribed Image Text:Review TConstants |PerIodic Tabie
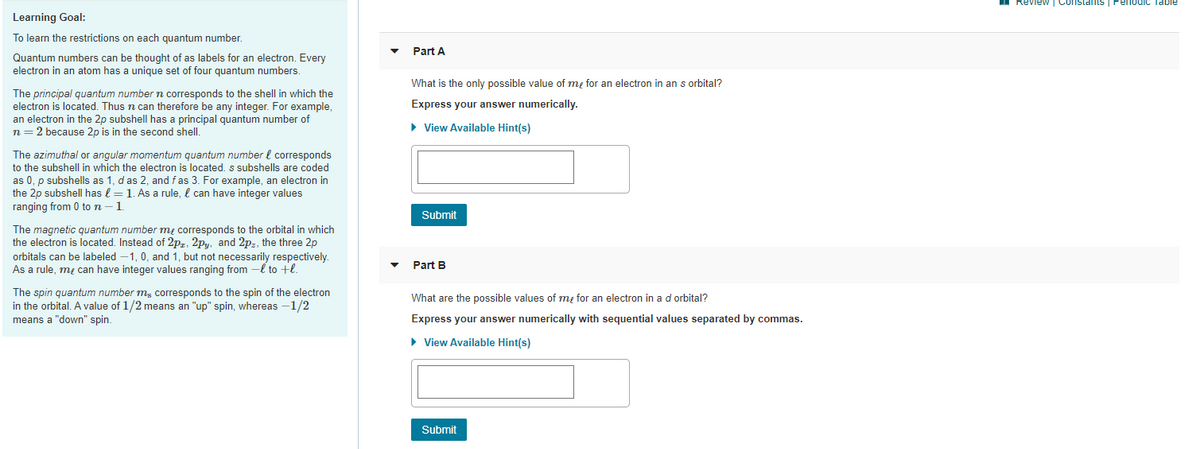
Learning Goal:
To learn the restrictions on each quantum number.
Part A
Quantum numbers can be thought of as labels for an electron. Every
electron in an atom has a unique set of four quantum numbers.
What is the only possible value of m, for an electron in an s orbital?
The principal quantum numbern corresponds to the shell in which the
electron is located. Thus n can therefore be any integer. For example,
an electron in the 2p subshell has a principal quantum number of
n = 2 because 2p is in the second shell.
Express your answer numerically.
• View Available Hint(s)
The azimuthal or angular momentum quantum number l corresponds
to the subshell in which the electron is located. s subshells are coded
as 0, p subshells as 1, d as 2, and fas 3. For example, an electron in
the 2p subshell has l = 1. As a rule, l can have integer values
ranging from 0 to n -1
Submit
The magnetic quantum number mų corresponds to the orbital in which
the electron is located. Instead of 2pz, 2py, and 2pz, the three 2p
orbitals can be labeled –1, 0, and 1, but not necessarily respectively.
As a rule, me can have integer values ranging from -l to +l.
Part B
The spin quantum number m, corresponds to the spin of the electron
in the orbital. A value of 1/2 means an "up" spin, whereas -1/2
means a "down" spin.
What are the possible values of me for an electron in a d orbital?
Express your answer numerically with sequential values separated by commas.
• View Available Hint(s)
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning