Review ormation about an electron's probable be represented by an electron-density an orbital. An orbital is characterized tation in space. Part A What is the angular-momentum quantum number (also called the azimuthal quantum number), l, for the orbital shown here? Express your answer numerically as an integer. View Available Hint(s) l= Submit Part B V Compare the orbital shown in Parts A to the orbital shown here in size, shape, and orientation. Part B Compare the orbital shown in Parts A to the orbital shown here in size, shape, and orientation Z х Which quantum number(s) would be different for these two orbitals? View Available Hint(s) n only l only me only l and me п, е, and me
Review ormation about an electron's probable be represented by an electron-density an orbital. An orbital is characterized tation in space. Part A What is the angular-momentum quantum number (also called the azimuthal quantum number), l, for the orbital shown here? Express your answer numerically as an integer. View Available Hint(s) l= Submit Part B V Compare the orbital shown in Parts A to the orbital shown here in size, shape, and orientation. Part B Compare the orbital shown in Parts A to the orbital shown here in size, shape, and orientation Z х Which quantum number(s) would be different for these two orbitals? View Available Hint(s) n only l only me only l and me п, е, and me
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 28P: Quantum mechanics predicts that the energy of the ground state of the H atom is 13.6eV . Insight...
Related questions
Question
100%
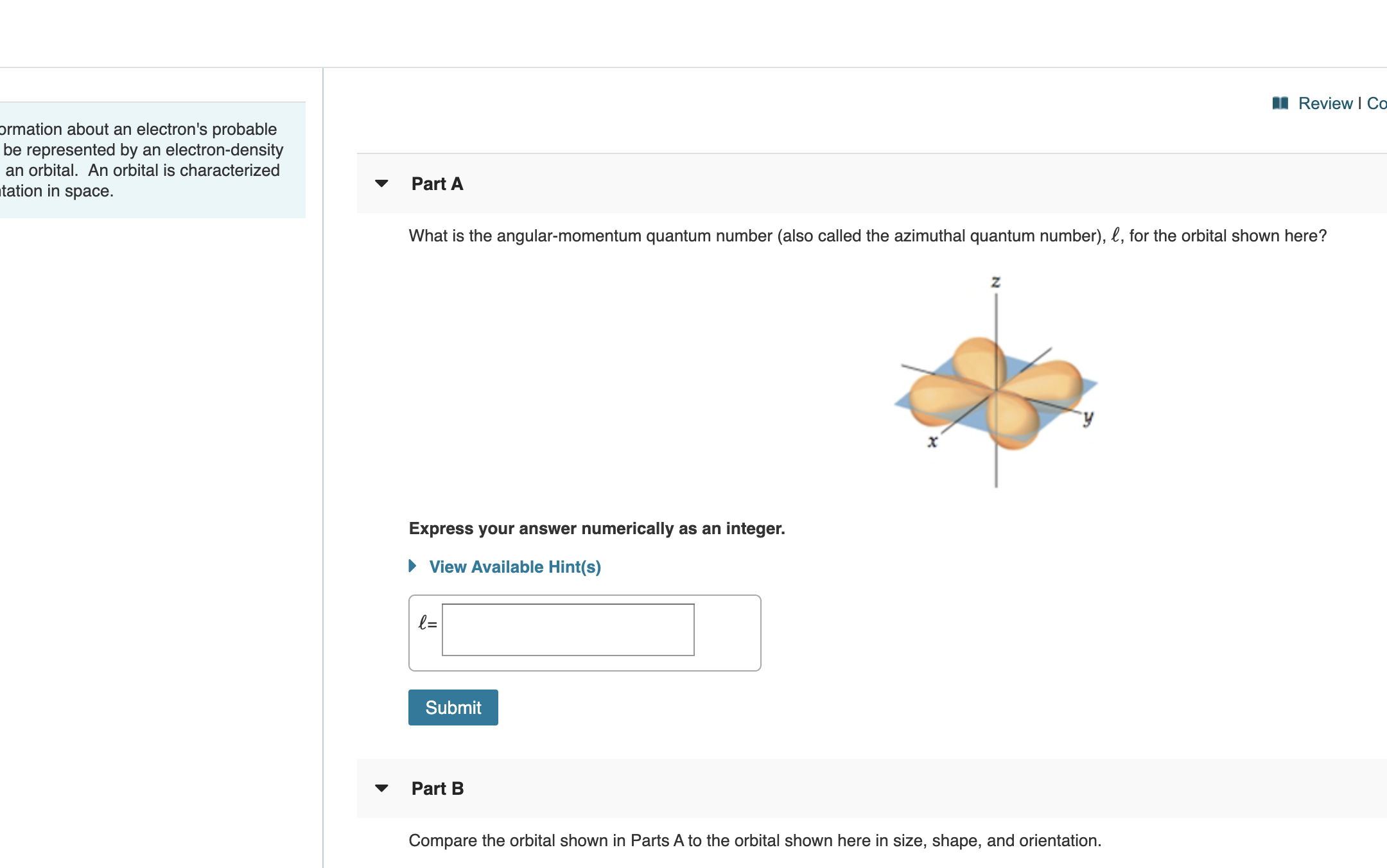
Transcribed Image Text:Review
ormation about an electron's probable
be represented by an electron-density
an orbital. An orbital is characterized
tation in space.
Part A
What is the angular-momentum quantum number (also called the azimuthal quantum number), l, for the orbital shown here?
Express your answer numerically as an integer.
View Available Hint(s)
l=
Submit
Part B
V
Compare the orbital shown in Parts A to the orbital shown here in size, shape, and orientation.
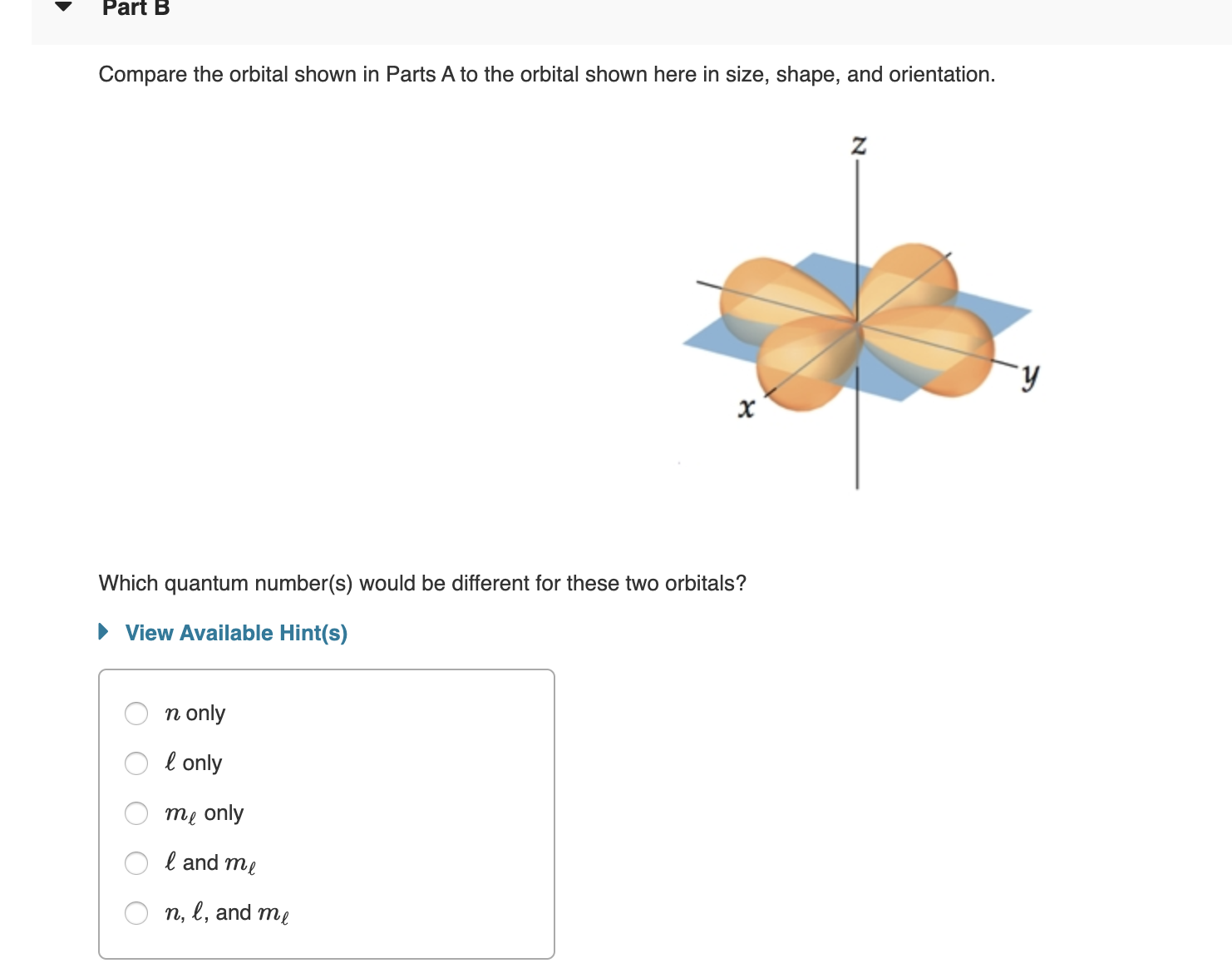
Transcribed Image Text:Part B
Compare the orbital shown in Parts A to the orbital shown here in size, shape, and orientation
Z
х
Which quantum number(s) would be different for these two orbitals?
View Available Hint(s)
n only
l only
me only
l and me
п, е, and me
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
