Chem Post Lab Questions 3. Given that accuracy is how close values are to an accepted value and precision refers to reproducibility, comment on the accuracy and precision of your experiment. Predict causes the errors with in the experiment? (Do NOT state human error.)
Chem Post Lab Questions 3. Given that accuracy is how close values are to an accepted value and precision refers to reproducibility, comment on the accuracy and precision of your experiment. Predict causes the errors with in the experiment? (Do NOT state human error.)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.8: Measuring Reaction Enthalpies: Calorimetry
Problem 4.9PSP
Related questions
Question
Please help with question 3
Transcribed Image Text:Chem 105-Online
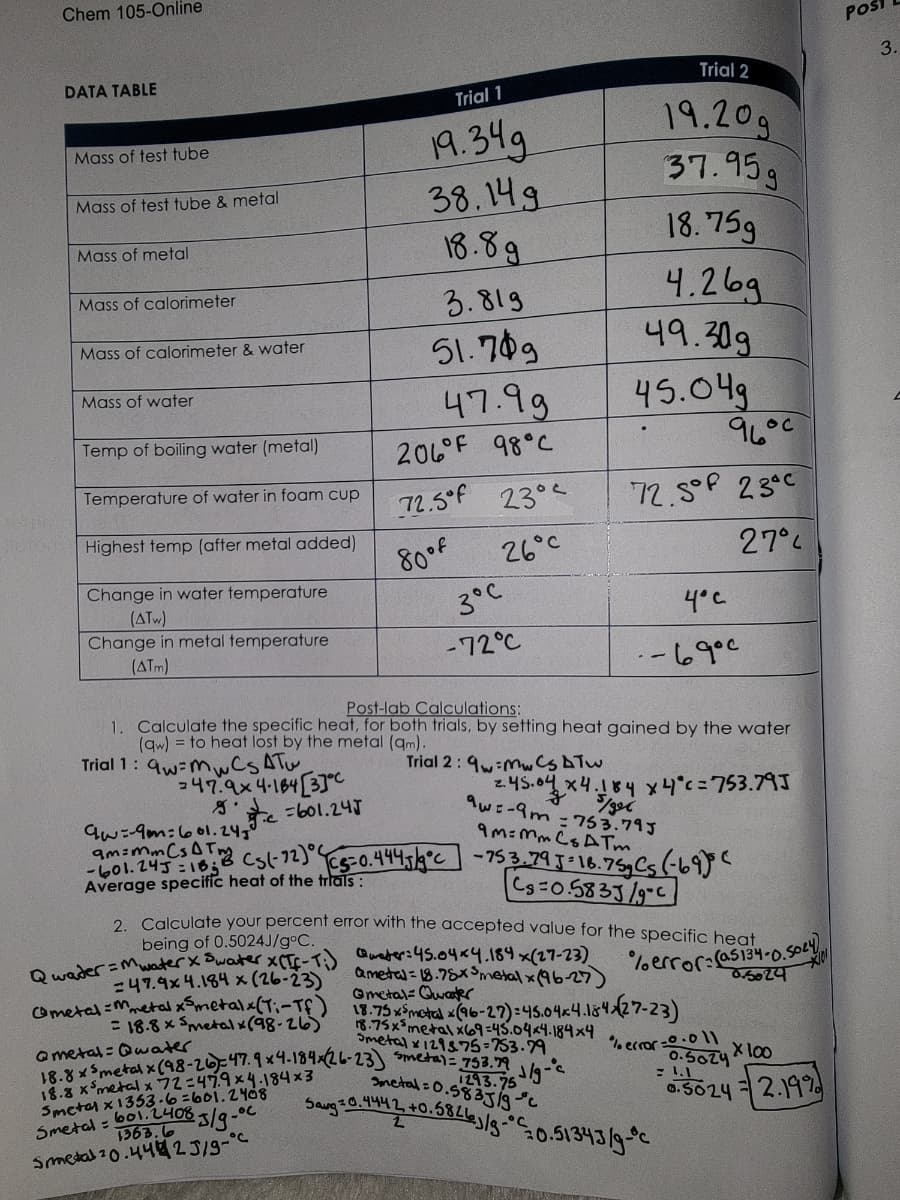
DATA TABLE
Mass of test tube
Mass of test tube & metal
Mass of metal
Mass of calorimeter
Mass of calorimeter & water
Mass of water
Temp of boiling water (metal)
Temperature of water in foam cup
Highest temp (after metal added)
Change in water temperature
(ATW)
Change in metal temperature
(ATM)
Trial 1
19.34g
38.14g
18.89
3.819
51.749
247.9x4-184[37°C
47.99
206°F 98°C
72.5°F 23°
80 of
26°C
3°C
-72°C
Trial 2
19.20g
37.95g
18.75g
4.26g
49.309
45.04g
१८००
72.5° 23°C
27%
Trial 2:9-MCS A Tw
Post-lab Calculations:
1. Calculate the specific heat, for both trials, by setting heat gained by the water
(qw) = to heat lost by the metal (am).
Trial 1: qw-mwCS ATW,
4°C
۹۰۰ ما - -
2.45.04x4.184 x 4°c=753.79J
9w:-9m=753.79J
9M-Mm Cs ATm
c=601.245
9w=-9m=601.24⁰
am=ms (s(-12) (5=0.4445/9°C -753.79 J-16.75g Cs (-69) (
Cg=0.5833/9°C
Average specific heat of the trials:
2. Calculate your percent error with the accepted value for the specific heat
being of 0.5024J/gºC.
Qwader = Mwater x Swater x (Tf-T:)
47.9x 4.184 x (26-23)
Cmetal Metal xSmetalx (Ti-Tf)
= 18.8 x 5metal x (98-26)
@metal: Qwater
Quater: 45.04x4.184 x(27-23)
&meta=18.78x Smetal x(96-27)
@metal-Qwater
%error: (as134-0.5024
0.5024
18.75 x5 metal (96-27):45.04x4.184x(27-23)
18.75x5metal x69=45.04x4.184x4
Smetal x129375-753.79
18.8x5metal x (98-26)=47.9x4-184x (26-23) Smetal = 753.79 1/9-c
18.8 x ³metal x 72=479x4.184x3
Smetal x 1353-6=601.2408
metal=0.5835/gfc
1293.75
Smetal = 6012.4083/g.c
Smetal 20.442 5/9-
-°C
% error -0.011
Saug 20.4442 +0.58263/g-0.51343/9-c
0.5ozy 100
0.5024 = 2.19%
Posl
3.

Transcribed Image Text:Chem
Post Lab Questions
Given that
accuracy is how close values are to an accepted value and
precision refers to reproducibility, comment on the accuracy and precision of
your experiment. Predict causes the errors with in the experiment? (Do NOT
state human error.)
C
3.
4. Suppose you took the test tube out of the boiling water bath for ~30 seconds as
you forgot to take the initial temperature of the water in the calorimeter. How
would your specific heat calculation be affected for your metal? How do you
know? (Be specific, do not simply state the value would be "different", "off" or
"incorrect".)
5. Suppose a student took the test tube of metal and placed it directly into the cup
of water instead of actually pouring the metal in the cup. How would this
impact the results of the experiment? (Be specific, do not simply state the value
would be "different", "off" or "incorrect". Hint, think of what else is being added
to the calorimeter).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER