Chemical Equation REDOX Atom Atom Yes/No Oxidized Reduced Reducing Oxidizing Agent Agent +2 -2 +2.5 -2 -1 Yes I S2032- I2 S2032- (aq) + I2 (ag) >S4O6²- (nq) + I- (aq). Nag + Clag ->NaCle 2. Al) + Cu²*(ag) –> Al³* (ag) + Cu») [aq] 3. H21) + F2i) -> HF1. 4. SiO21) + Ca) -> Si) + COg). 5. Pb (NO3)2 (mg) + 2KIag) -> Pbl2) +2KNO3(aq) 1.
Chemical Equation REDOX Atom Atom Yes/No Oxidized Reduced Reducing Oxidizing Agent Agent +2 -2 +2.5 -2 -1 Yes I S2032- I2 S2032- (aq) + I2 (ag) >S4O6²- (nq) + I- (aq). Nag + Clag ->NaCle 2. Al) + Cu²*(ag) –> Al³* (ag) + Cu») [aq] 3. H21) + F2i) -> HF1. 4. SiO21) + Ca) -> Si) + COg). 5. Pb (NO3)2 (mg) + 2KIag) -> Pbl2) +2KNO3(aq) 1.
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 10RQ
Related questions
Question
100%
This is only one activity and therefore, this should be considered as a single question po. Sana hindi katulad sa ibang experts dito na nagtatake advantage kasi di na pwedeng mabawi yung question. Sana masagot lahat. Salamat!
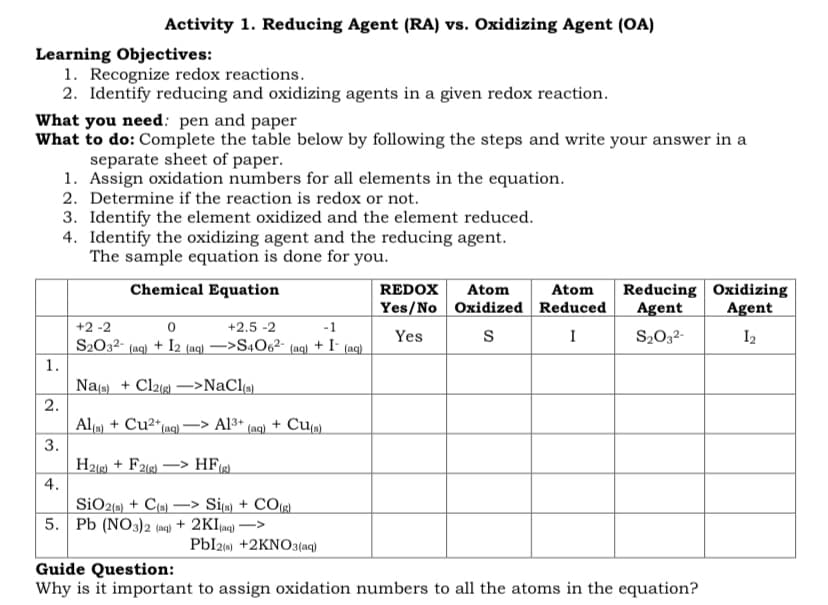
Transcribed Image Text:Activity 1. Reducing Agent (RA) vs. Oxidizing Agent (OA)
Learning Objectives:
1. Recognize redox reactions.
2. Identify reducing and oxidizing agents in a given redox reaction.
What you need: pen and paper
What to do: Complete the table below by following the steps and write your answer in a
separate sheet of paper.
1. Assign oxidation numbers for all elements in the equation.
2. Determine if the reaction is redox or not.
3. Identify the element oxidized and the element reduced.
4. Identify the oxidizing agent and the reducing agent.
The sample equation is done for you.
Chemical Equation
REDOX
Atom
Atom
Reducing Oxidizing
Yes/No Oxidized Reduced
Agent
Agent
+2 -2
+2.5 -2
-1
S2032- (ac) + I2 (ac) –>S4O6²-
+ I-
Yes
I
S2032-
I2
(aq)
(aq)
1.
Nas) + Cl2ig) –>NaCl
2.
Al () + Cu2*(aq)–> Al3* (aq) + Cu(s).
H21) + F21g) *
4.
SiO2{) + C9) –> Si« + CO(g).
5. Pb (NO3)2 (mg) + 2KI(ag) ->
Pbl2) +2KNO3(aq)
Guide Question:
Why is it important to assign oxidation numbers to all the atoms in the equation?
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning