Using data from the table below, place the following in order of decreasing strength as oxidizing agents (all under standard conditions). Half-reaction E° (V) Fe+ +e + Fe+ Fe?+ + 2e + Fe O2 + 4H+ + 4e + 2H2O Ce+ +e- + Ce+ Cl2 + 2e 2CI Zn+ + 2e Zn 0.77 -0.44 1.23 1.70 1.36 -0.76 Decreasing of strength as oxidizing agents Drag and drop your selection from the following list to complete the answer: O2 Zn2+ Ce+ Fe+ Fe2+ Cl2
Using data from the table below, place the following in order of decreasing strength as oxidizing agents (all under standard conditions). Half-reaction E° (V) Fe+ +e + Fe+ Fe?+ + 2e + Fe O2 + 4H+ + 4e + 2H2O Ce+ +e- + Ce+ Cl2 + 2e 2CI Zn+ + 2e Zn 0.77 -0.44 1.23 1.70 1.36 -0.76 Decreasing of strength as oxidizing agents Drag and drop your selection from the following list to complete the answer: O2 Zn2+ Ce+ Fe+ Fe2+ Cl2
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 58E: Using data from Table 17-1, place the following in order of increasing strength as reducing agents...
Related questions
Question
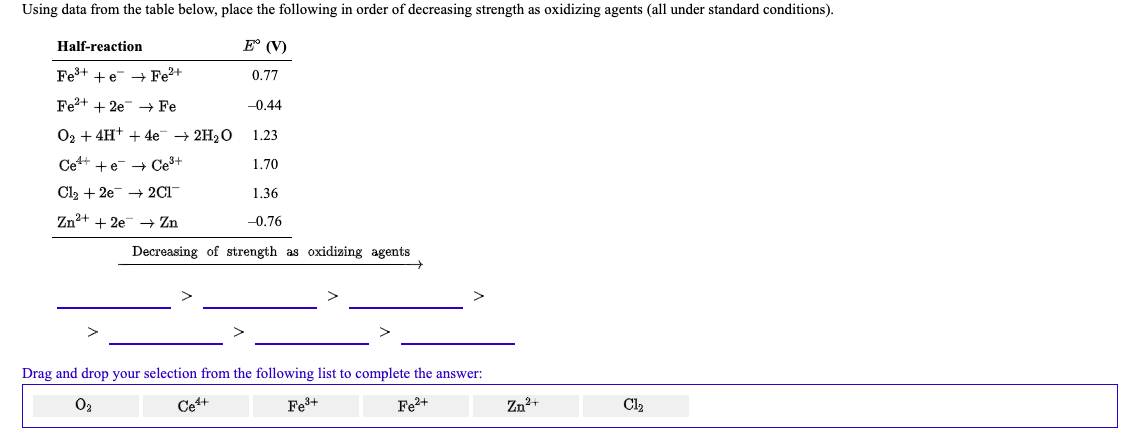
Transcribed Image Text:Using data from the table below, place the following in order of decreasing strength as oxidizing agents (all under standard conditions).
Half-reaction
E° (V)
Fe+ +e + Fe+
Fe?+ + 2e + Fe
O2 + 4H+ + 4e + 2H2O
Ce+ +e- + Ce+
Cl2 + 2e 2CI
Zn+ + 2e Zn
0.77
-0.44
1.23
1.70
1.36
-0.76
Decreasing of strength as oxidizing agents
Drag and drop your selection from the following list to complete the answer:
O2
Zn2+
Ce+
Fe+
Fe2+
Cl2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning