Consider the following half-reactions: Half-reaction E° (V) F2(g) + 2e → 2F°(aq) 2.870V Co*(aq) + 2e¯ → Co(s) -0.28oV C *(aq) + 3e¯ → Cr(s) -0.740V (1) The weakest oxidizing agent is: enter formula (2) The strongest reducing agent is: (3) The strongest oxidizing agent is: (4) The weakest reducing agent is: (5) Will Cr(s) reduce F2(g) to F(aq)? [ (6) Which species can be oxidized by Co²*(aq)? If none, leave box blank.
Consider the following half-reactions: Half-reaction E° (V) F2(g) + 2e → 2F°(aq) 2.870V Co*(aq) + 2e¯ → Co(s) -0.28oV C *(aq) + 3e¯ → Cr(s) -0.740V (1) The weakest oxidizing agent is: enter formula (2) The strongest reducing agent is: (3) The strongest oxidizing agent is: (4) The weakest reducing agent is: (5) Will Cr(s) reduce F2(g) to F(aq)? [ (6) Which species can be oxidized by Co²*(aq)? If none, leave box blank.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 105QAP: Consider a voltaic cell in which the following reaction occurs. Zn(s)+Sn2+(aq)Zn2+(aq)+Sn(s) (a)...
Related questions
Question
100%
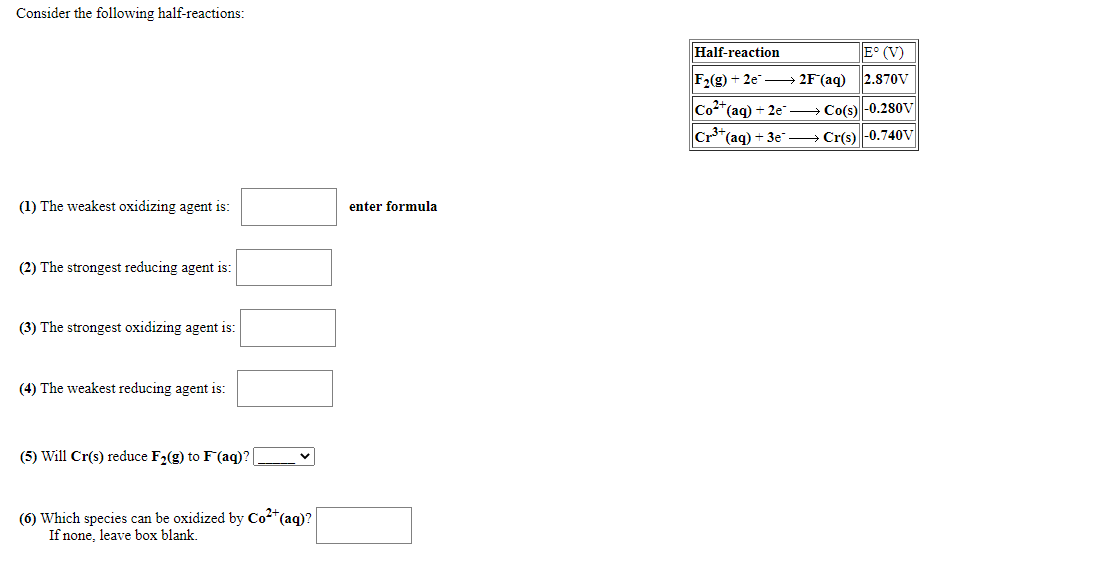
Transcribed Image Text:Consider the following half-reactions:
Half-reaction
E° (V)
F2(g) + 2e → 2F°(aq)
2.870V
Co**(aq) + 2e → Co(s) -0.280V
(aq) + 3e → Cr(s) -0.740V
(1) The weakest oxidizing agent is:
enter formula
(2) The strongest reducing agent is:
(3) The strongest oxidizing agent is:
(4) The weakest reducing agent is:
(5) Will Cr(s) reduce F2(g) to F(aq)?|
(6) Which species can be oxidized by Co*(aq)?
If none, leave box blank.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning