Chemical Soluble in oil Conductivity when Melting Boiling point dissolved in water point 152 °C Not conductive PF CF PCI AIF3 No 151 °C F184 °C Not conductive Not conductive Conductive Yes | 198 °C No - 94 °C 76 °C No 1291 °C Using principles of bonding and states of matter, which is the best constructed argument about which of the below would best dissolve in room temperature water and why? PF3 would dissolve best in polar water at room temperature because it is a polar molecule (polar molecules dissolve in polar molecules) and it is a liquid at room temperature and liquids dissolve better in water. CF4 would dissolve best in polar water at room temperature. It dissolves in oil so it must be a nonpolar molecule and nonpolar molecules dissolve better in polar molecules. In addition, it is a gas and gasses dissolve better in water. PCI3 would dissolve best in polar water at room temperature because it does not dissolve in oil which makes it a polar compound (polar compounds dissolve in polar compounds) and it is a liquid at room temperature and liquids dissolve better in water than gases FRRE CIear
Chemical Soluble in oil Conductivity when Melting Boiling point dissolved in water point 152 °C Not conductive PF CF PCI AIF3 No 151 °C F184 °C Not conductive Not conductive Conductive Yes | 198 °C No - 94 °C 76 °C No 1291 °C Using principles of bonding and states of matter, which is the best constructed argument about which of the below would best dissolve in room temperature water and why? PF3 would dissolve best in polar water at room temperature because it is a polar molecule (polar molecules dissolve in polar molecules) and it is a liquid at room temperature and liquids dissolve better in water. CF4 would dissolve best in polar water at room temperature. It dissolves in oil so it must be a nonpolar molecule and nonpolar molecules dissolve better in polar molecules. In addition, it is a gas and gasses dissolve better in water. PCI3 would dissolve best in polar water at room temperature because it does not dissolve in oil which makes it a polar compound (polar compounds dissolve in polar compounds) and it is a liquid at room temperature and liquids dissolve better in water than gases FRRE CIear
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.87QE
Related questions
Question
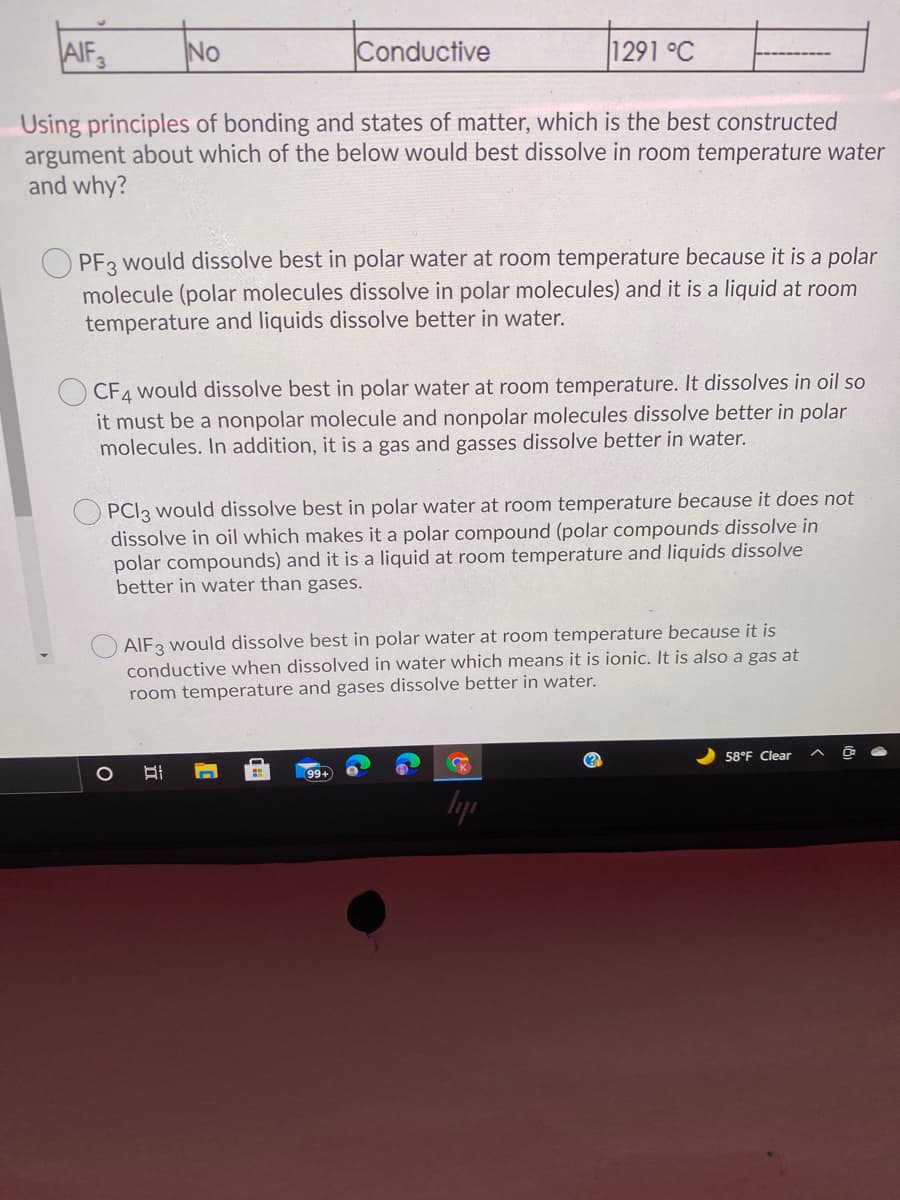
Transcribed Image Text:AIF3
No
Conductive
1291 °C
Using principles of bonding and states of matter, which is the best constructed
argument about which of the below would best dissolve in room temperature water
and why?
PF3 would dissolve best in polar water at room temperature because it is a polar
molecule (polar molecules dissolve in polar molecules) and it is a liquid at room
temperature and liquids dissolve better in water.
CF4 would dissolve best in polar water at room temperature. It dissolves in oil so
it must be a nonpolar molecule and nonpolar molecules dissolve better in polar
molecules. In addition, it is a gas and gasses dissolve better in water.
PCI3 would dissolve best in polar water at room temperature because it does not
dissolve in oil which makes it a polar compound (polar compounds dissolve in
polar compounds) and it is a liquid at room temperature and liquids dissolve
better in water than gases.
AIF3 would dissolve best in polar water at room temperature because it is
conductive when dissolved in water which means it is ionic. It is also a gas at
room temperature and gases dissolve better in water.
58°F Clear
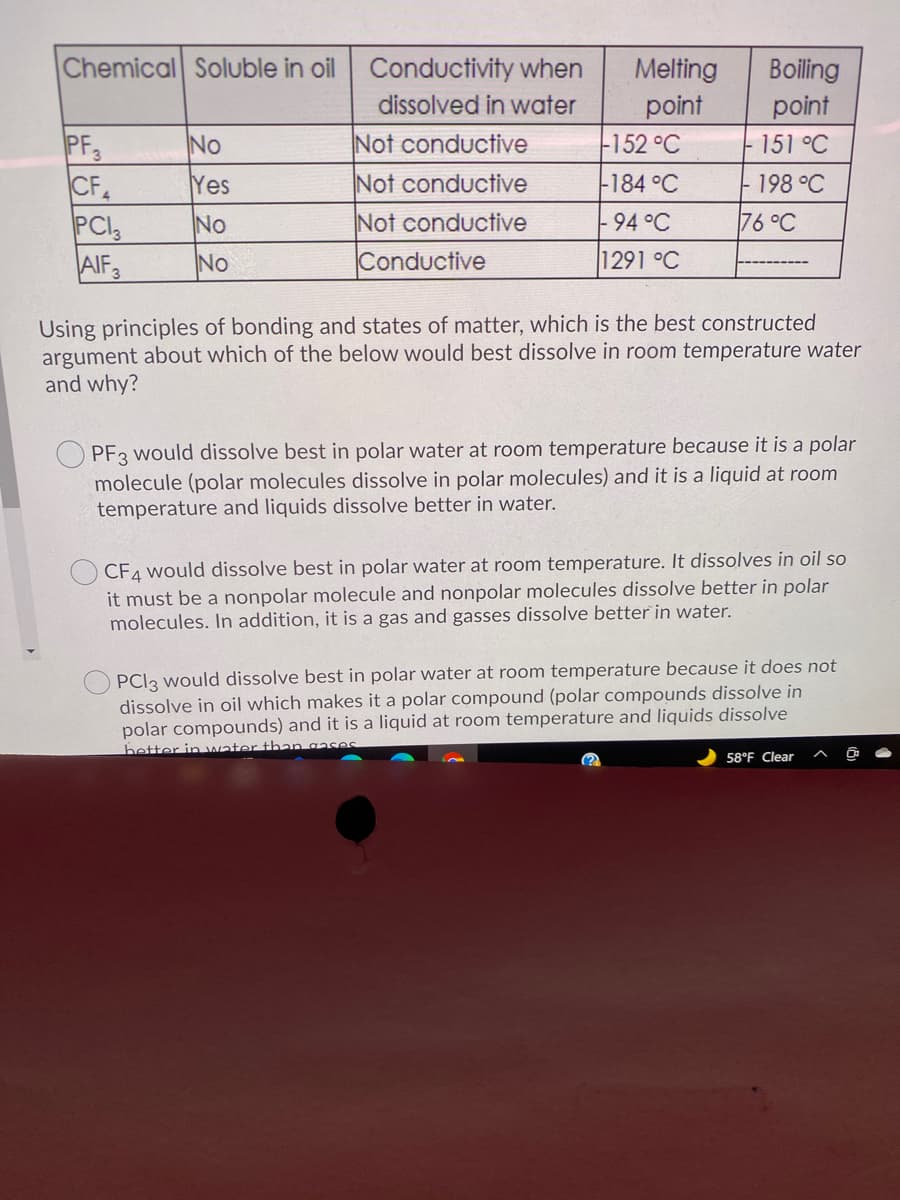
Transcribed Image Text:Chemical Soluble in oil Conductivity when
Melting
point
-152 °C
Boiling
point
dissolved in water
PF3
CF
PCI
AIF3
Not conductive
Not conductive
Not conductive
Conductive
No
151 °C
Yes
-184 °C
-198 °C
No
94°C
76 °C
No
1291 °C
Using principles of bonding and states of matter, which is the best constructed
argument about which of the below would best dissolve in room temperature water
and why?
O PF3 would dissolve best in polar water at room temperature because it is a polar
molecule (polar molecules dissolve in polar molecules) and it is a liquid at room
temperature and liquids dissolve better in water.
CF4 would dissolve best in polar water at room temperature. It dissolves in oil so
it must be a nonpolar molecule and nonpolar molecules dissolve better in polar
molecules. In addition, it is a gas and gasses dissolve better in water.
PCI3 would dissolve best in polar water at room temperature because it does not
dissolve in oil which makes it a polar compound (polar compounds dissolve in
polar compounds) and it is a liquid at room temperature and liquids dissolve
better in water than gases
58°F Clear
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning