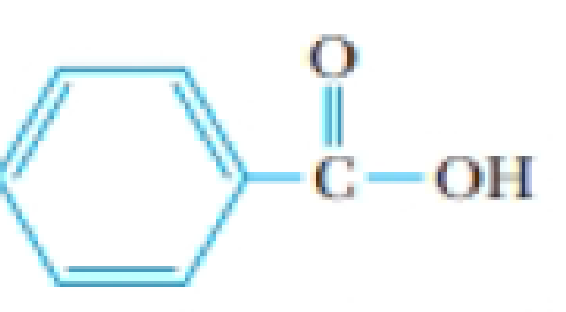
Problem 1PS: You dissolve 2.56 g of succinic acid, C2H4(CO2H)2, in 500. mL of water. Assuming that the density of... Problem 2PS: You dissolve 45.0 g of camphor, C10H16O, in 425 mL of ethanol, C2H5OH. Calculate the molality, mole... Problem 3PS Problem 4PS Problem 5PS Problem 6PS Problem 7PS Problem 8PS Problem 9PS: Hydrochloric acid is sold as a concentrated aqueous solution. If the concentration of commercial HCl... Problem 10PS: Concentrated sulfuric acid has a density of 1.84 g/cm3 and is 95.0% by weight H2SO4. What is the... Problem 11PS: The average lithium ion concentration in seawater is 0.18 ppm. What is the molality of Li+ in... Problem 12PS: Silver ion has an average concentration of 28 ppb (parts per billion) in U.S. water supplies. (a)... Problem 13PS: Which pairs of liquids will be miscible? (a) H2O and CH3CH2CH2CH3 (b) C6H6 (benzene) and CCl4 (c)... Problem 14PS: Acetone, CH3COCH3, is quite soluble in water. Explain why this should be so. Problem 15PS Problem 16PS: Use the following data to calculate the enthalpy of solution of sodium perchlorate, NaClO4:... Problem 17PS: You make a saturated solution of NaCl at 25 C. No solid is present in the beaker holding the... Problem 18PS: Some lithium chloride, LiCl, is dissolved in 100 mL of water in one beaker, and some Li2SO4 is... Problem 19PS Problem 20PS: The Henrys law constant for O2 in water at 25 is given in Table 13.2. Which of the following is a... Problem 21PS: An unopened soda can has an aqueous CO2 concentration of 0.0506 m at 25 C. What is the pressure of... Problem 22PS: Hydrogen gas has a Henrys law constant of 7.8 104 mol/kgbar at 25 C when dissolving in water. If... Problem 23PS: A sealed flask contains water and oxygen gas at 25 C. The O2 gas has a partial pressure of 1.5 atm.... Problem 24PS: Butane, C4H10, has been suggested as the refrigerant in household compressors such as those found in... Problem 25PS: A 35.0-g sample of ethylene glycol, HOCH2CH2OH, is dissolved in 500.0 g of water. The vapor pressure... Problem 26PS: Urea, (NH2)2CO, which is widely used in fertilizers and plastics, is quite soluble in water. If you... Problem 27PS: Pure ethylene glycol, HOCH2CH2OH, is added 2.00 kg of water in the cooling system of a car. The... Problem 28PS: Pure iodine (105 g) is dissolved in 325 g of CCl4 at 65 C. Given that the vapor pressure of CCl4 at... Problem 29PS Problem 30PS: What is the boiling point of a solution composed of 15.0 g of urea, (NH2)2CO, in 0.500 kg of water? Problem 31PS Problem 32PS Problem 33PS Problem 34PS: Some ethylene glycol, HOCH2CH2OH, is added to your cars cooling system along with 5.0 kg of water.... Problem 35PS: You dissolve 15.0 g of sucrose, C12H22O11, in a cup of water (225 g). What is the freezing point of... Problem 36PS: A typical bottle of wine consists of an 11% solution (by weight) of ethanol (C2H5OH) in water. If... Problem 37PS Problem 38PS: Estimate the osmotic pressure of human blood at 37 C. Assume blood is isotonic with a 0.154 M NaCl... Problem 39PS: An aqueous solution containing 1.00 g of bovine insulin (a protein, not ionized) per liter has an... Problem 40PS: Calculate the osmotic pressure of a 0.0120 M solution of NaCl in water at 0 C. Assume the vant Hoff... Problem 41PS: You add 0.255 g of an orange, crystalline compound whose empirical formula is C10H8Fe to 11.12 g of... Problem 42PS: Butylated hydroxyanisole (BHA) is used in margarine and other fats and oils. (It is used as an... Problem 43PS: Benzyl acetate is one of the active components of oil of jasmine. If 0.125 g of the compound is... Problem 44PS: Anthracene, a hydrocarbon obtained from coal, has an empirical formula of C7H5. To find its... Problem 45PS: An aqueous solution contains 0.180 g of an unknown, nonionic solute in 50.0 g of water. The solution... Problem 46PS: Aluminon, an organic compound, is used as a reagent to test for the presence of the aluminum ion in... Problem 47PS Problem 48PS: To make homemade ice cream, you cool the milk and cream by immersing the container in ice and a... Problem 49PS: List the following aqueous solutions in order of increasing melting point. (The last three are all... Problem 50PS: Arrange the following aqueous solutions in order of decreasing freezing point. (The last three are... Problem 51PS: When solutions of BaCl2 and Na2SO4 are mixed, the mixture becomes cloudy. After a few days, a white... Problem 52PS: The dispersed phase of a certain colloidal dispersion consists of spheres of diameter 1.0 102 nm.... Problem 53GQ: Phenylcarbinol is used in nasal sprays as a preservative. A solution of 0.52 g of the compound in... Problem 54GQ: (a) Which aqueous solution is expected to have the higher boiling point: 0.10 m Na2SO4 or 0.15 m... Problem 55GQ: Arrange the following aqueous solutions in order of (i) increasing vapor pressure of water and (ii)... Problem 56GQ Problem 57GQ: Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a reagent to precipitate nickel ion. Assume that 53.0... Problem 58GQ: A 10.7 m solution of NaOH has a density of 1.33 g/cm3 at 20 C. Calculate the following: (a) the mole... Problem 59GQ: Concentrated aqueous ammonia has a molarity of 14.8 mol/L and a density of 0.90 g/cm3. What is the... Problem 60GQ Problem 61GQ: If you want a solution that is 0.100 m in ions, what mass of Na2SO4 must you dissolve in 125 g of... Problem 62GQ: Consider the following aqueous solutions: (i) 0.20 m HOCH2CH2OH (nonvolatile, nonelectrolyte); (ii)... Problem 63GQ: (a) Which solution is expected to have the higher boiling point: 0.20 m KBr or 0.30 m sugar? (b)... Problem 64GQ: The solubility of NaCl in water at 100 C is 39.1 g/100. g of water Calculate the boiling point of... Problem 65GQ: Instead of using NaCl to melt the ice on your sidewalk you decide to use CaCl2. If you add 35.0 g of... Problem 66GQ: The smell of ripe raspberries is due to 4-(p-hydroxyphenyl)-2-butanone, which has the empirical... Problem 67GQ: Hexachlorophene has been used in germicidal soap. What is its molar mass if 0.640 g of the compound,... Problem 68GQ: The solubility of ammonium formate, NH4CHO2, in 100. g of water is 102 g at 0 C and 546 g at 80 C. A... Problem 69GQ: How much N2 can dissolve in water at 25 C if the N2 partial pressure is 585 mm Hg? Problem 70GQ: Cigars are best stored in a humidor at 18 C and 55% relative humidity. This means the pressure of... Problem 71GQ: An aqueous solution containing 10.0 g of starch per liter has an osmotic pressure of 3.8 mm Hg at 25... Problem 72GQ Problem 73GQ: Calculate the enthalpies of solution for Li2SO4 and K2SO4. Are the solution processes exothermic or... Problem 74GQ: Water at 25 C has a density of 0.997 g/cm3. Calculate the molality and molarity of pure water at... Problem 75GQ: If a volatile solute is added to a volatile solvent, both substances contribute to the vapor... Problem 76GQ: A solution is made by adding 50.0 mL of ethanol (C2H5OH, d = 0.789 g/mL) to 50.0 mL of water (d =... Problem 77GQ: A 2.0% (by mass) aqueous solution of novocainium chloride (C13H21ClN2O2) freezes at 0.237 C.... Problem 78GQ: A solution is 4.00% (by mass) maltose and 96.00% water. It freezes at 0.229 C. (a) Calculate the... Problem 79GQ: The following table lists the concentrations of the principal ions in seawater: (a) Calculate the... Problem 80GQ: A tree is 10.0 m tall. (a) What must be the total molarity of the solutes if sap rises to the top of... Problem 81GQ Problem 82GQ: A compound is known to be a potassium halide, KX. If 4.00 g of the salt is dissolved in exactly 100... Problem 85GQ Problem 86GQ: If one is very careful, it is possible to float a needle on the surface of water. (If the needle is... Problem 87IL: A solution of benzoic acid in benzene has a freezing point of 3.1 C and a boiling point of 82.6 C.... Problem 88IL: You dissolve 5.0 mg of iodine, I2, in 25 mL of water. You then add 10.0 mL of CCl4 and shake the... Problem 89IL Problem 90IL: In a police forensics lab, you examine a package that may contain heroin. However, you find the... Problem 91IL: An organic compound contains carbon (71.17%), hydrogen (5.12%) with the remainder nitrogen.... Problem 92IL Problem 93SCQ: When sails of Mg2+, Ca2+, and Be2+ are placed in water, the positive ion is hydrated (as is the... Problem 94SCQ: Explain why a cucumber shrivels up when it is placed in a concentrated solution of salt. Problem 95SCQ Problem 96SCQ: A 100.-gram sample of sodium chloride (NaCl) is added to 100. mL of water at 0 C. After equilibrium... Problem 97SCQ Problem 98SCQ Problem 99SCQ: Starch contains CC, CH, CO, and OH bonds. Hydrocarbons have only CC and CH bonds. Both starch and... Problem 100SCQ Problem 101SCQ: You have two aqueous solutions separated by a semipermeable membrane. One contains 5.85 g of NaCl... Problem 102SCQ Problem 104SCQ: Sodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride... Problem 105SCQ Problem 106SCQ Problem 107SCQ format_list_bulleted


 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




