Here is a sketch of a 3d, orbital: This sketch is about 1800 pm wide. The coordinate (x, y, and z) axes are also shown. point A You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse. point B finding the electron at point A, compares to PR, the probability of finding the electron at point B. 200 pm below the nucleus along the -z axis. A' Suppose an atom with its nucleus at the origin has an electron in a 3d., orbital. Complete each row of the table below by deciding how P compare PA to PB < P A. В 200 pm behind the nucleus, along the +y axis. : P. B %3D oP=P3 200 pm to the left of the nucleus, along the -x axis. 200 pm to the right of the nucleus, along the +x axis. OP, > P, В P PB P < P. A. = P В A. MacBook Air Submit As F6 O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
Here is a sketch of a 3d, orbital: This sketch is about 1800 pm wide. The coordinate (x, y, and z) axes are also shown. point A You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse. point B finding the electron at point A, compares to PR, the probability of finding the electron at point B. 200 pm below the nucleus along the -z axis. A' Suppose an atom with its nucleus at the origin has an electron in a 3d., orbital. Complete each row of the table below by deciding how P compare PA to PB < P A. В 200 pm behind the nucleus, along the +y axis. : P. B %3D oP=P3 200 pm to the left of the nucleus, along the -x axis. 200 pm to the right of the nucleus, along the +x axis. OP, > P, В P PB P < P. A. = P В A. MacBook Air Submit As F6 O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter6: Electronic Structure And Periodic Properties Of Elements
Section: Chapter Questions
Problem 41E: Consider the orbitals shown here in outline. (a) What is the maximum number of electrons contained...
Related questions
Question
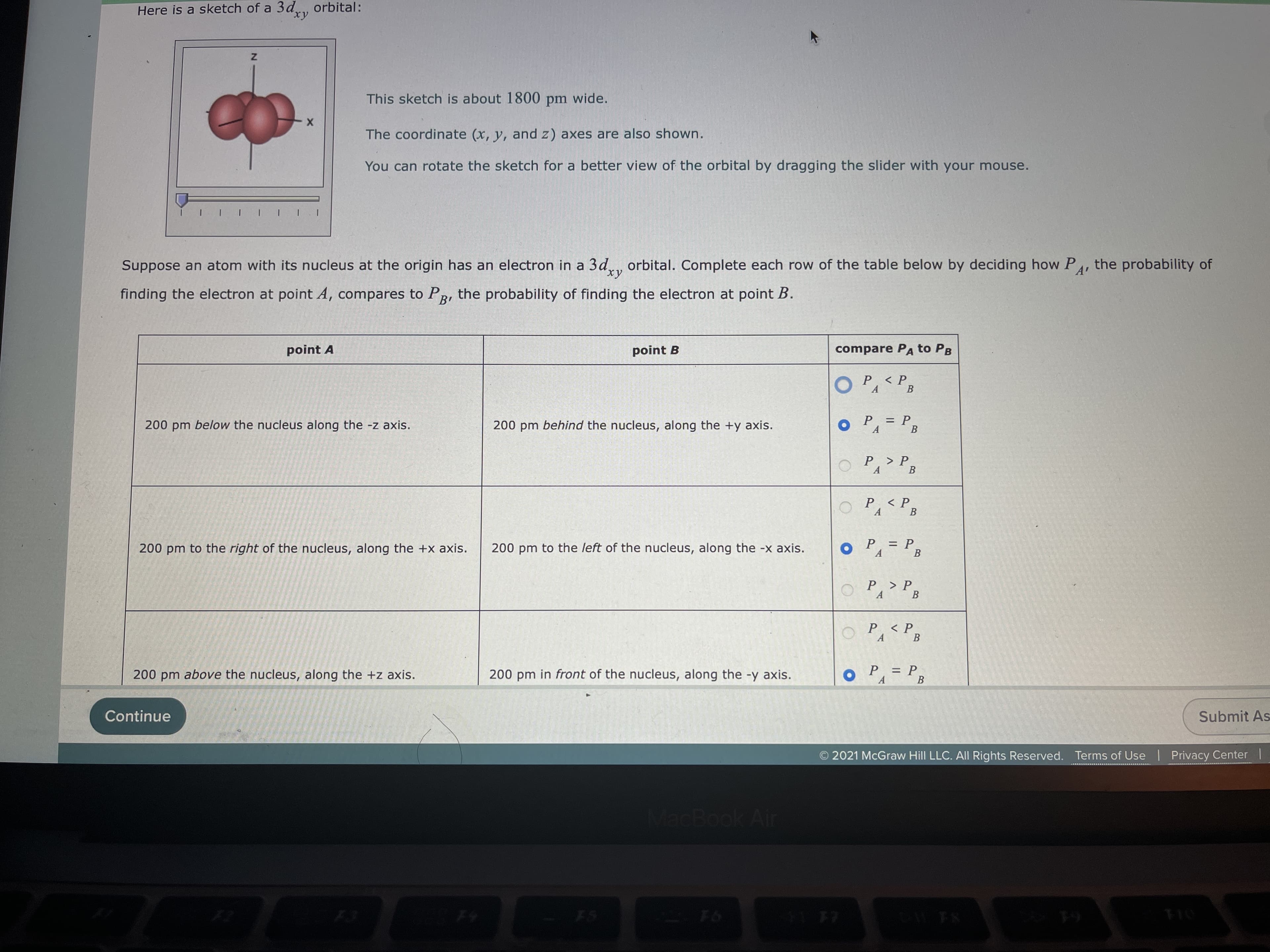
Transcribed Image Text:Here is a sketch of a 3d, orbital:
This sketch is about 1800 pm wide.
The coordinate (x, y, and z) axes are also shown.
point A
You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse.
point B
finding the electron at point A, compares to PR, the probability of finding the electron at point B.
200 pm below the nucleus along the -z axis.
A'
Suppose an atom with its nucleus at the origin has an electron in a 3d., orbital. Complete each row of the table below by deciding how P
compare PA to PB
< P
A.
В
200 pm behind the nucleus, along the +y axis.
: P.
B
%3D
oP=P3
200 pm to the left of the nucleus, along the -x axis.
200 pm to the right of the nucleus, along the +x axis.
OP, > P,
В
P <P
Continue
A.
В
A
200 pm above the nucleus, along the +z axis.
B.
200 pm in front of the nucleus, along the -y axis.
Pa> PB
P < P.
A.
= P
В
A.
MacBook Air
Submit As
F6
O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning