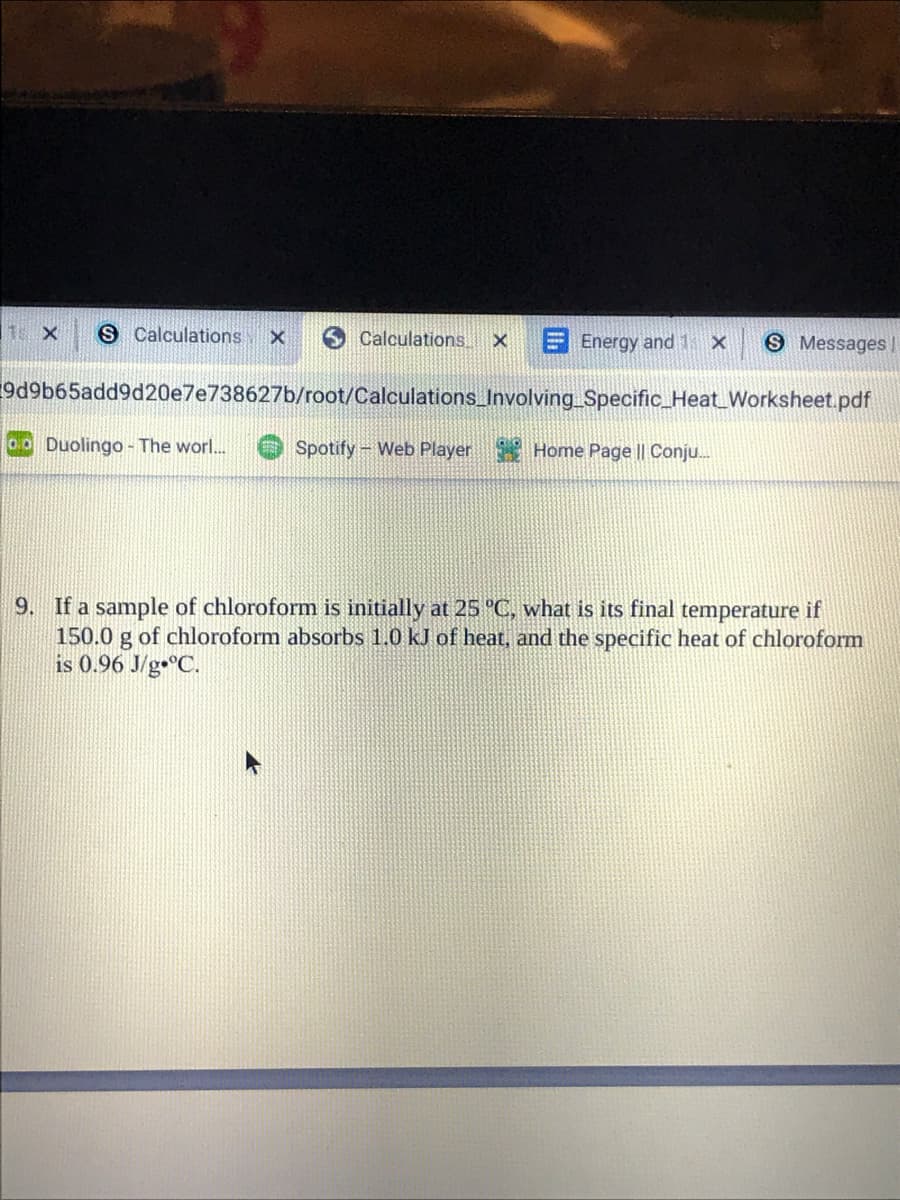
S Calculations Calculations. EEnergy and 1 x S Messages| 9d9b65add9d20e7e738627b/root/Calculations_Involving Specific_Heat_Worksheet.pdf o0 Duolingo - The worl.. Spotify- Web Player Home Page || Conju. 9. If a sample of chloroform is initially at 25 °C, what is its final temperature if 150.0 g of chloroform absorbs 1.0 kJ of heat, and the specific heat of chloroform is 0.96 J/g•°C.
S Calculations Calculations. EEnergy and 1 x S Messages| 9d9b65add9d20e7e738627b/root/Calculations_Involving Specific_Heat_Worksheet.pdf o0 Duolingo - The worl.. Spotify- Web Player Home Page || Conju. 9. If a sample of chloroform is initially at 25 °C, what is its final temperature if 150.0 g of chloroform absorbs 1.0 kJ of heat, and the specific heat of chloroform is 0.96 J/g•°C.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.34E: Aballoon filled with 0.505 mole of gascontracts reversibly from 1.0L to 0.10 L ata constant...
Related questions
Question
Transcribed Image Text:S Calculations
Calculations.
EEnergy and 1 x
S Messages|
9d9b65add9d20e7e738627b/root/Calculations_Involving Specific_Heat_Worksheet.pdf
o0 Duolingo - The worl..
Spotify- Web Player Home Page || Conju.
9. If a sample of chloroform is initially at 25 °C, what is its final temperature if
150.0 g of chloroform absorbs 1.0 kJ of heat, and the specific heat of chloroform
is 0.96 J/g•°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning