nucleus örbits nucleus Rutherford Model Bohr Model The diagram shows Rutherford's atomic model and Bohr's revision of that model. What evidence caused Bohr to modify Rutherford's theory of atomic structure? А Radioactive decay indicates that atoms are not the smallest particles of matter The formation of ionic compounds indicates that electrons may transfer from one atom to another Save/Exit 20 21 22 23
nucleus örbits nucleus Rutherford Model Bohr Model The diagram shows Rutherford's atomic model and Bohr's revision of that model. What evidence caused Bohr to modify Rutherford's theory of atomic structure? А Radioactive decay indicates that atoms are not the smallest particles of matter The formation of ionic compounds indicates that electrons may transfer from one atom to another Save/Exit 20 21 22 23
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section: Chapter Questions
Problem 6CR: What is meant by anuclear atom? Describe the points of Ruth erford’s model for the nuclear atom and...
Related questions
Question
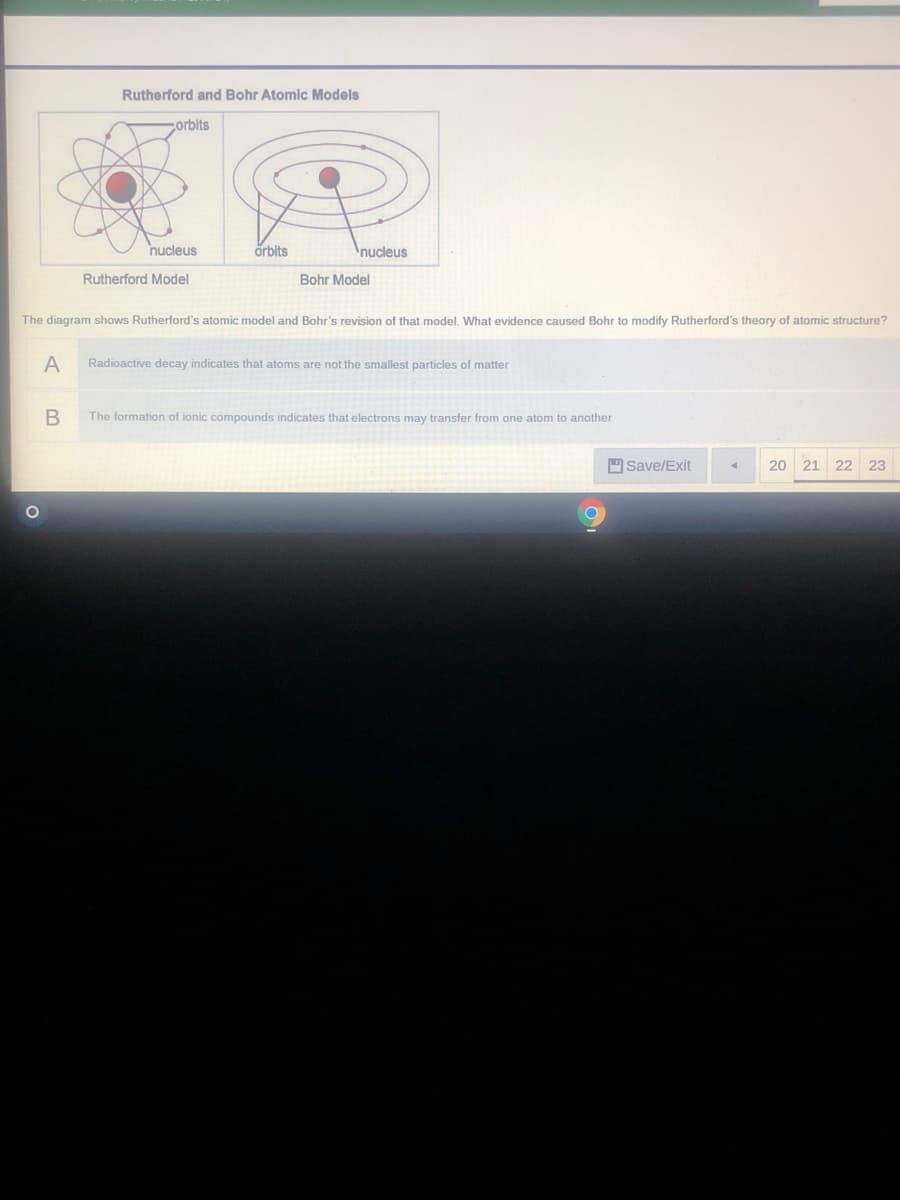
Transcribed Image Text:Rutherford and Bohr Atomic Models
corbits
nucleus
örbits
nucleus
Rutherford Model
Bohr Model
The diagram shows Rutherford's atomic model and Bohr's revision of that model, What evidence caused Bohr to modify Rutherford's theory of atomic structure?
Radioactive decay indicates that atoms are not the smallest particles of matter
The formation of ionic compounds indicates that electrons may transfer from one atom to another
Save/Exit
20
21 22 23
Transcribed Image Text:A
Radioactive decay indicates that atoms are not the smallest particles of matter
The formation of ionic compounds indicates that electrons may transfer from one atom to another
C
Measurements indicate that different atoms have different masses
Light emissions indicate that atoms have multiple energy levels.
Save
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
