240 220 200 NACIO3 180 KNO 160 140 KBrt 120 100 80 60 NaCl 40 20 20 40 60 100 120 Temperature (°C) 1. Which of these statements is true according to the graph shown above? a. More KNO, will dissolve than KBr at 0°C. b. Temperature has little effect on the water solubility of NaCl. c. KBr is insoluble in water at low temperatures. d. An increase in temperature causes a decrease in the solubility of NacIO, in water. 2. At 40°C, 100 grams of NaCIO, is added to 100 grams of water. The solution would be... a. saturated b. unsaturated C. saturated with excess d. supersaturated Solubility (g/100 g of water)
240 220 200 NACIO3 180 KNO 160 140 KBrt 120 100 80 60 NaCl 40 20 20 40 60 100 120 Temperature (°C) 1. Which of these statements is true according to the graph shown above? a. More KNO, will dissolve than KBr at 0°C. b. Temperature has little effect on the water solubility of NaCl. c. KBr is insoluble in water at low temperatures. d. An increase in temperature causes a decrease in the solubility of NacIO, in water. 2. At 40°C, 100 grams of NaCIO, is added to 100 grams of water. The solution would be... a. saturated b. unsaturated C. saturated with excess d. supersaturated Solubility (g/100 g of water)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 61E: Which of the following will have the lowest total vapor pressure at 25C? a. pure water (vapor...
Related questions
Question
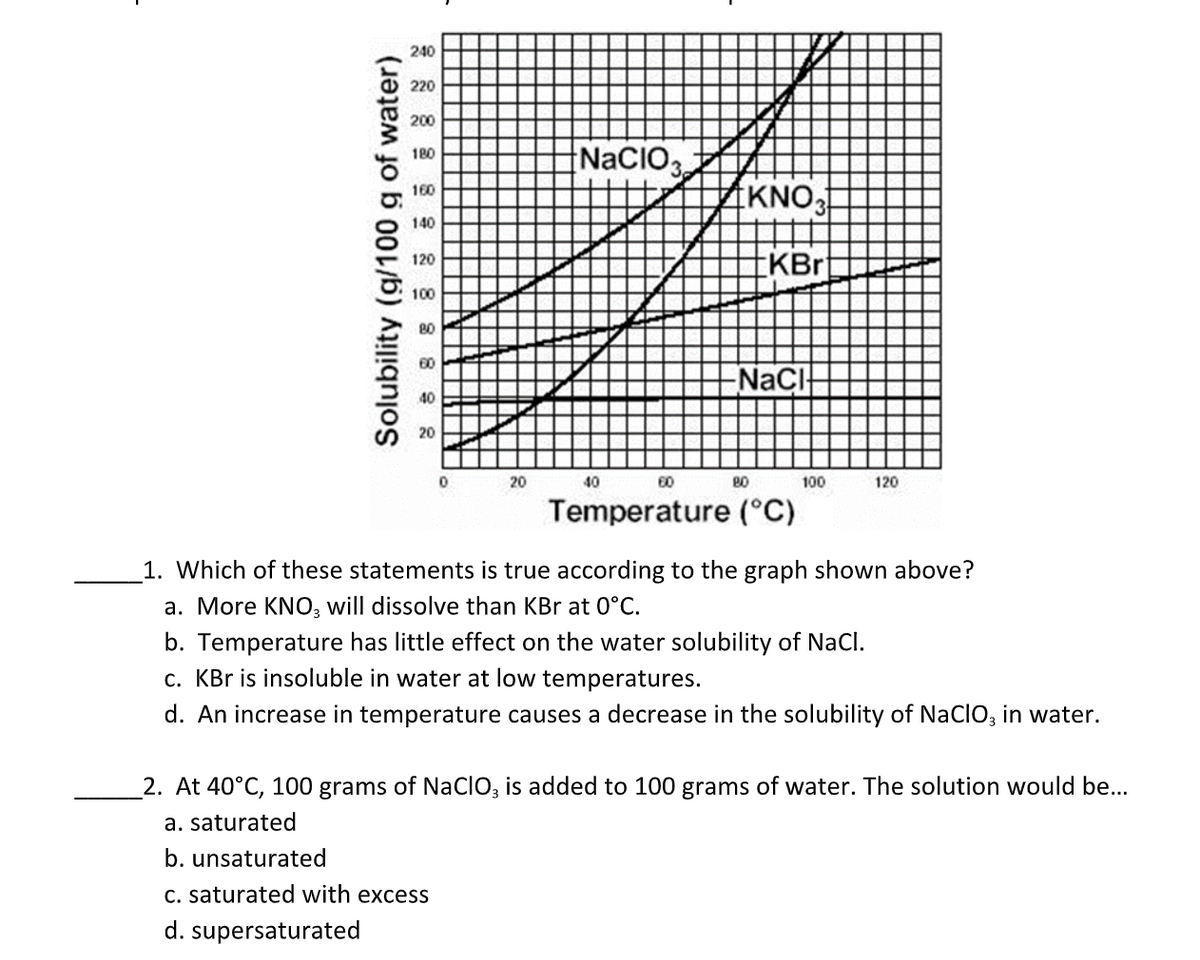
Transcribed Image Text:240
220
200
NACIO3
180
KNO
160
140
KBrt
120
100
80
60
NaCl
40
20
20
40
60
100
120
Temperature (°C)
1. Which of these statements is true according to the graph shown above?
a. More KNO, will dissolve than KBr at 0°C.
b. Temperature has little effect on the water solubility of NaCl.
c. KBr is insoluble in water at low temperatures.
d. An increase in temperature causes a decrease in the solubility of NacIO, in water.
2. At 40°C, 100 grams of NaCIO, is added to 100 grams of water. The solution would be...
a. saturated
b. unsaturated
C. saturated with excess
d. supersaturated
Solubility (g/100 g of water)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning