क Rb ALEKS Chemistry- 2021FA-CHI X A ALEKS - Griffin Barden - Learn eks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-JcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4EDeRİLhu6AbUmc3VVdLPcASZ3o67Wr4xcXIZmcLfpPf_PLSp4nj4wgn0SQA3P0?1oBw7QYjlI O THERMOCHEMISTRY Understanding the definitions of heat and work A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The 1 atm pressure cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) The temperature of the water bath is monitored, and it is determined from this data that 259. kJ of heat flows into the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the piston does 322. kJ of work on the system during the reaction. gases exothermic Is the reaction exothermic or endothermic? endothermic dn O Does the temperature of the water bath go up or down? UMop O O neither u! O no O neither Does the piston move in or out? O absorb Does the reaction absorb or release energy? O release O neither How much energy does the reaction absorb or release? Round your answer to 3 significant digits. Explanation Check 2021 McGraw Hill LLC. All Rights Reserved. Terms of
क Rb ALEKS Chemistry- 2021FA-CHI X A ALEKS - Griffin Barden - Learn eks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-JcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4EDeRİLhu6AbUmc3VVdLPcASZ3o67Wr4xcXIZmcLfpPf_PLSp4nj4wgn0SQA3P0?1oBw7QYjlI O THERMOCHEMISTRY Understanding the definitions of heat and work A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The 1 atm pressure cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) The temperature of the water bath is monitored, and it is determined from this data that 259. kJ of heat flows into the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the piston does 322. kJ of work on the system during the reaction. gases exothermic Is the reaction exothermic or endothermic? endothermic dn O Does the temperature of the water bath go up or down? UMop O O neither u! O no O neither Does the piston move in or out? O absorb Does the reaction absorb or release energy? O release O neither How much energy does the reaction absorb or release? Round your answer to 3 significant digits. Explanation Check 2021 McGraw Hill LLC. All Rights Reserved. Terms of
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Transcribed Image Text:क
Rb ALEKS Chemistry- 2021FA-CHI X
A ALEKS - Griffin Barden - Learn
eks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-JcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4EDeRİLhu6AbUmc3VVdLPcASZ3o67Wr4xcXIZmcLfpPf_PLSp4nj4wgn0SQA3P0?1oBw7QYjlI
O THERMOCHEMISTRY
Understanding the definitions of heat and work
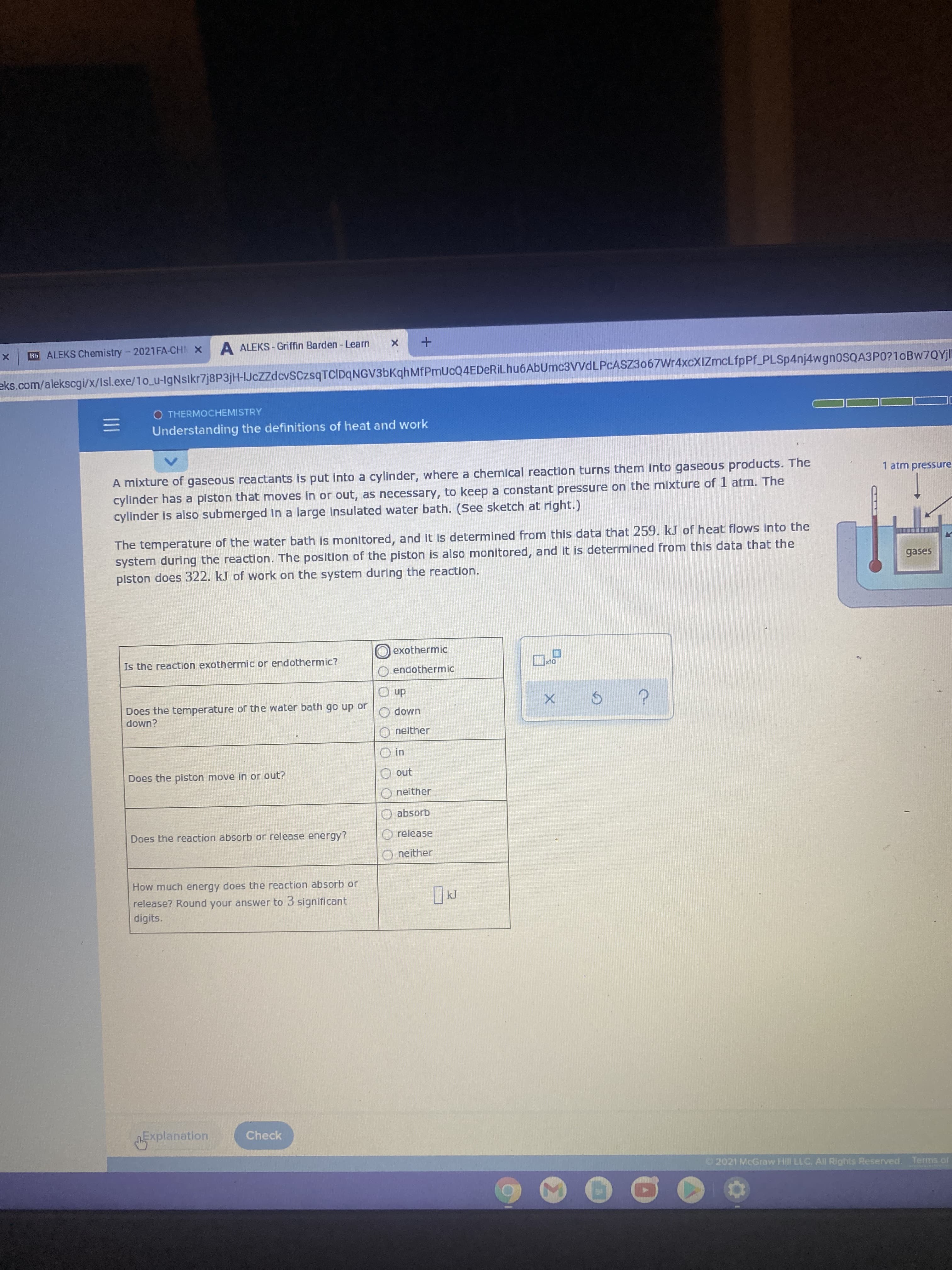
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The
1 atm pressure
cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The
cylinder is also submerged in a large insulated water bath. (See sketch at right.)
The temperature of the water bath is monitored, and it is determined from this data that 259. kJ of heat flows into the
system during the reaction. The position of the piston is also monitored, and it is determined from this data that the
piston does 322. kJ of work on the system during the reaction.
gases
exothermic
Is the reaction exothermic or endothermic?
endothermic
dn O
Does the temperature of the water bath go up or
down?
UMop O
O neither
u! O
no O
neither
Does the piston move in or out?
O absorb
Does the reaction absorb or release energy?
O release
O neither
How much energy does the reaction absorb or
release? Round your answer to 3 significant
digits.
Explanation
Check
2021 McGraw Hill LLC. All Rights Reserved. Terms of
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning