EPVR7vQaBdOTkdkkqbUQV8RO00PTsppl-RQ1FDU2MTMCYHQ/viewform?hr_submission=Chkig t-Wikip... Algebra Foundatio.. Image result for sur.. 2 Classwork 2.3 Eukaryotic Cels Democritus, Dalton, Rutherford Which of the following is NOT a part of John Dalton's model of the atom? * 7 points O Atoms of the same element are all alike in mass, shape, and size Atoms of two elements may combine in different ratios to form more than one compound Elements are composed of microscopic, indivisible particles that we call atoms Atoms are made of parts that have a positive charge and a negative charge Atoms of different elements have different masses and sizes The union of two or more atoms of different elements forms chemical compounds Atoms combine to form compounds in small, whole number ratios such as 1:1, 2:2, 2:3, and so on
EPVR7vQaBdOTkdkkqbUQV8RO00PTsppl-RQ1FDU2MTMCYHQ/viewform?hr_submission=Chkig t-Wikip... Algebra Foundatio.. Image result for sur.. 2 Classwork 2.3 Eukaryotic Cels Democritus, Dalton, Rutherford Which of the following is NOT a part of John Dalton's model of the atom? * 7 points O Atoms of the same element are all alike in mass, shape, and size Atoms of two elements may combine in different ratios to form more than one compound Elements are composed of microscopic, indivisible particles that we call atoms Atoms are made of parts that have a positive charge and a negative charge Atoms of different elements have different masses and sizes The union of two or more atoms of different elements forms chemical compounds Atoms combine to form compounds in small, whole number ratios such as 1:1, 2:2, 2:3, and so on
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:e tap to activate Page 7 of 7 Page 6 of 7
iz #3 Ana
Classwork for 5 Chemistry 5
E Quiz - The Atom
EPVR7vQaBdOTkdkkqbUQV8RO00PTsppl-RQ1FDU2MTMCYHQ/viewform?hr_submission=Chkig
t-Wikip.
A Algebra Foundatio.
G Image result for sur.
Classwork
2.3 Eukaryotic Cells
Democritus, Dalton, Rutherford
Which of the following is NOT a part of John Dalton's model of the atom? * 7 points
O Atoms of the same element are all alike in mass, shape, and size
Atoms of two elements may combine in different ratios to form more than one
compound
Elements are composed of microscopic, indivisible particles that we call atoms
Atoms are made of parts that have a positive charge and a negative charge
Atoms of different elements have different masses and sizes
O The union of two or more atoms of different elements forms chemical compounds
Atoms combine to form compounds in small, whole number ratios such as 1:1, 2:2,
2:3, and so on
Which of the following was one of Empedocles elements (from ancient
Greece)?
points
Transcribed Image Text:LSfxPvR7vQaBdOTkdkkqbUQV8RO00PTsppl-RQ1FDU2MTMCYHQ/viewform?hr_submis
ic drift - Wikip..
Algebra Foundatio.
Image result for sur..
Classwork
G2.3 EL
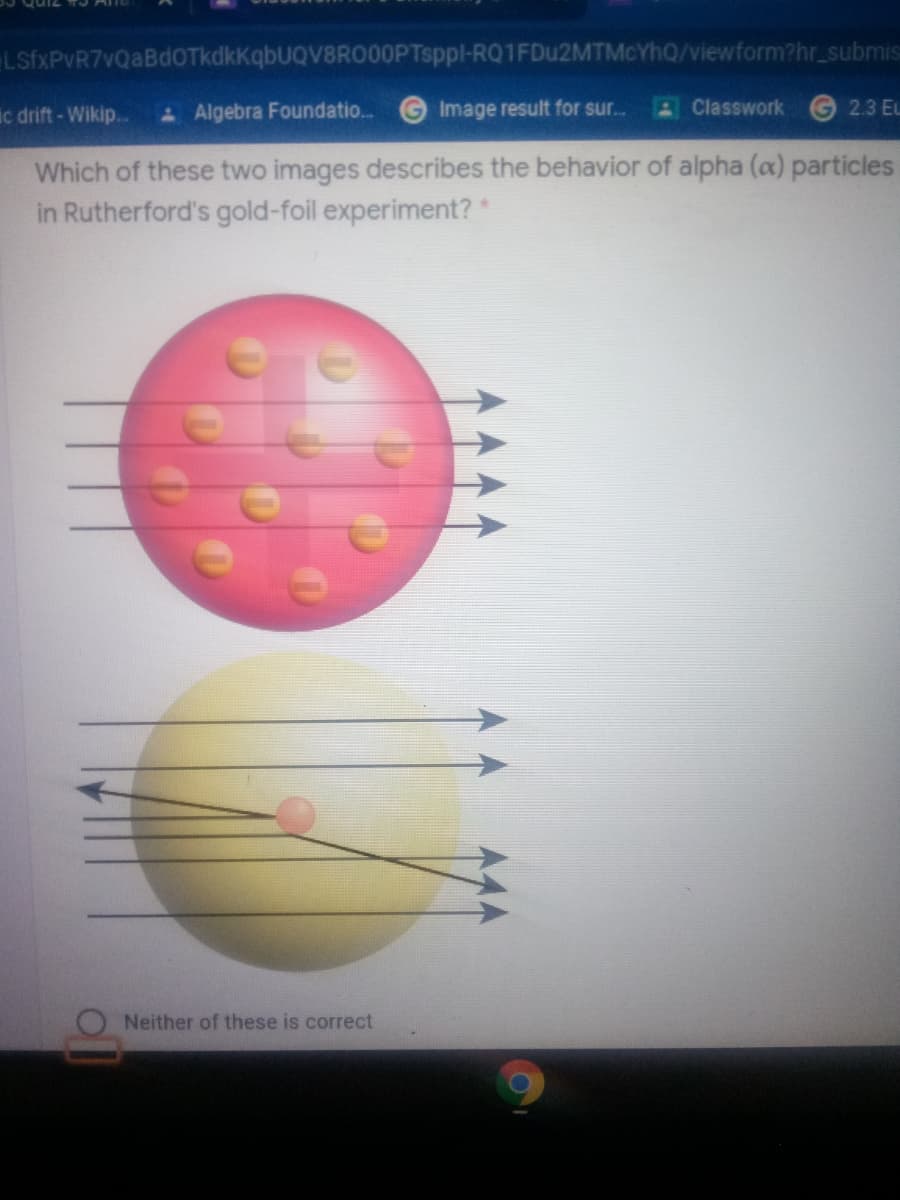
Which of these two images describes the behavior of alpha (a) particles
in Rutherford's gold-foil experiment? *
Neither of these is correct
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY