Aluminum can replace copper ions in solution. Use the following table to answer this item. Reaction E° (V) Reaction E (V) F2 + 2e¯ –→ 2F¯ 2.866 Cu²+ + e¯ → Cu+ 0.153 4+ 2+ H2O2 + 2H* + 2e¯ → 2H2O 1.776 Sn + 2e¯ → Sn“ 0.151 AgBr + e 2H* + 2e¯ –→ H2 N20 + 2H* + 2e¯ → N2 + H,O 1.766 → Ag + Br 0.07133 Au+ + e¯ –→ Au 1.692 0.0000 MnO4 + 4H† + 3e¯ → MnO2 + 2H3O 1.679 Fe3+ + 3e¯ → Fe -0.037 2D* + 2e¯ –→ D2 -0.044 HСIO, + H* + Зе — Cl2 + 2H,O Mn+ + e¯ → Mn²+ 1.63 Pb2+ + 2e¯ → Pb -0.1262 Sn²+ + 2e¯ → Sn -0.1375 1.5415 Ni?+ + 2e¯ → Ni -0.257 MnO4 + 8H* + 5e° Mn²+ + 4H2O 1.507 Co²+ + 2e¯ → Co -0.28 PBSO4 + 2e¯ → Pb + SO4²¬ Cr+ + e¯ → Cr²+ -0.3588 Au³+ + 3e¯ → Au 1.498 -0.407 Cl2 + 2e¯ → 2 Cl¯ O2 + 4H† + 4e 1.358 Fe2+ + 2e¯ → Fe -0.447 → 2H2O 1.229 Cr³* + 3e¯ → Cr -0.744 Br2 + 2e 2H9²+ + 2e¯ → Hg2² Hg²+ + 2e Ag* + e¯ → Ag Hg, → 2Br 1.087 Zn²+ + 2e¯ → Zn -0.7618 2+ 0.920 2H2O + 2e¯ → H2 + 20H- Cr2+ + 2e¯ → Cr -0.8277 → Hg 0.851 -0.913 0.7996 Al³+ + 3e¯ –→ Al -1.662 2+ + 2e → 2Hg 0.7973 Be2+ + 2e¯ → Be -1.847 Fe+ + e → Fe2+ 0.771 Н, + 2е — 2H -2.23 MnO4 + e¯ → MnO4²- 0.558 Mg²+ + 2e¯ –→ Mg - 2.372 I3 + 2e¯ -→ 3I¯ 0.536 Na* + e –→ Na -2.71 I2 + 2e → 21- 0.5355 Ca2+ + 2e¯ → Ca - 2.868 Cu* + e¯ –→ Cu 0.521 Lit + e → Li - 3.04 O2 + 2H2O + 4e¯ → 40H- Cu²+ + 2e 0.401 → Cu 0.3419 Hg,Cl, + 2e¯ → 2Hg + 2CI- 0.26828 AgCl + e¯ → Ag + Cl¯ 0.22233 True O False
Aluminum can replace copper ions in solution. Use the following table to answer this item. Reaction E° (V) Reaction E (V) F2 + 2e¯ –→ 2F¯ 2.866 Cu²+ + e¯ → Cu+ 0.153 4+ 2+ H2O2 + 2H* + 2e¯ → 2H2O 1.776 Sn + 2e¯ → Sn“ 0.151 AgBr + e 2H* + 2e¯ –→ H2 N20 + 2H* + 2e¯ → N2 + H,O 1.766 → Ag + Br 0.07133 Au+ + e¯ –→ Au 1.692 0.0000 MnO4 + 4H† + 3e¯ → MnO2 + 2H3O 1.679 Fe3+ + 3e¯ → Fe -0.037 2D* + 2e¯ –→ D2 -0.044 HСIO, + H* + Зе — Cl2 + 2H,O Mn+ + e¯ → Mn²+ 1.63 Pb2+ + 2e¯ → Pb -0.1262 Sn²+ + 2e¯ → Sn -0.1375 1.5415 Ni?+ + 2e¯ → Ni -0.257 MnO4 + 8H* + 5e° Mn²+ + 4H2O 1.507 Co²+ + 2e¯ → Co -0.28 PBSO4 + 2e¯ → Pb + SO4²¬ Cr+ + e¯ → Cr²+ -0.3588 Au³+ + 3e¯ → Au 1.498 -0.407 Cl2 + 2e¯ → 2 Cl¯ O2 + 4H† + 4e 1.358 Fe2+ + 2e¯ → Fe -0.447 → 2H2O 1.229 Cr³* + 3e¯ → Cr -0.744 Br2 + 2e 2H9²+ + 2e¯ → Hg2² Hg²+ + 2e Ag* + e¯ → Ag Hg, → 2Br 1.087 Zn²+ + 2e¯ → Zn -0.7618 2+ 0.920 2H2O + 2e¯ → H2 + 20H- Cr2+ + 2e¯ → Cr -0.8277 → Hg 0.851 -0.913 0.7996 Al³+ + 3e¯ –→ Al -1.662 2+ + 2e → 2Hg 0.7973 Be2+ + 2e¯ → Be -1.847 Fe+ + e → Fe2+ 0.771 Н, + 2е — 2H -2.23 MnO4 + e¯ → MnO4²- 0.558 Mg²+ + 2e¯ –→ Mg - 2.372 I3 + 2e¯ -→ 3I¯ 0.536 Na* + e –→ Na -2.71 I2 + 2e → 21- 0.5355 Ca2+ + 2e¯ → Ca - 2.868 Cu* + e¯ –→ Cu 0.521 Lit + e → Li - 3.04 O2 + 2H2O + 4e¯ → 40H- Cu²+ + 2e 0.401 → Cu 0.3419 Hg,Cl, + 2e¯ → 2Hg + 2CI- 0.26828 AgCl + e¯ → Ag + Cl¯ 0.22233 True O False
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 21Q: When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is...
Related questions
Question
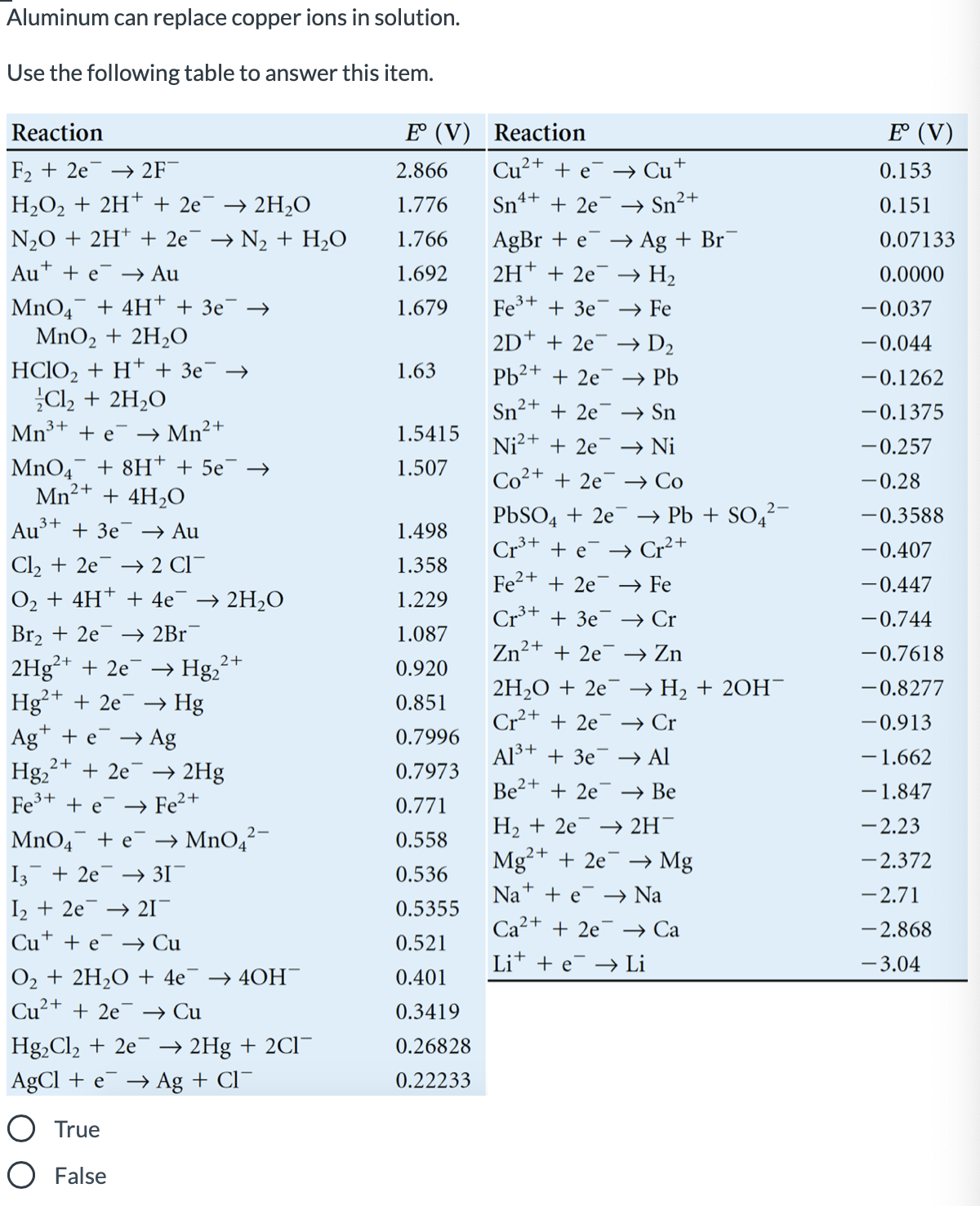
Transcribed Image Text:Aluminum can replace copper ions in solution.
Use the following table to answer this item.
Reaction
E° (V) Reaction
E (V)
F2 + 2e¯ –→ 2F¯
2.866
Cu²+ + e¯
→ Cu+
0.153
4+
2+
H2O2 + 2H* + 2e¯ → 2H2O
1.776
Sn
+ 2e¯ → Sn“
0.151
AgBr + e
2H* + 2e¯ –→ H2
N20 + 2H* + 2e¯ → N2 + H,O
1.766
→ Ag + Br
0.07133
Au+ + e¯ –→ Au
1.692
0.0000
MnO4 + 4H† + 3e¯ →
MnO2 + 2H3O
1.679
Fe3+ + 3e¯ → Fe
-0.037
2D* + 2e¯ –→ D2
-0.044
HСIO, + H* + Зе —
Cl2 + 2H,O
Mn+ + e¯ → Mn²+
1.63
Pb2+ + 2e¯ → Pb
-0.1262
Sn²+ + 2e¯ → Sn
-0.1375
1.5415
Ni?+ + 2e¯ → Ni
-0.257
MnO4 + 8H* + 5e°
Mn²+ + 4H2O
1.507
Co²+ + 2e¯ → Co
-0.28
PBSO4 + 2e¯ → Pb + SO4²¬
Cr+ + e¯ → Cr²+
-0.3588
Au³+ + 3e¯ → Au
1.498
-0.407
Cl2 + 2e¯ → 2 Cl¯
O2 + 4H† + 4e
1.358
Fe2+ + 2e¯ → Fe
-0.447
→ 2H2O
1.229
Cr³* + 3e¯ → Cr
-0.744
Br2 + 2e
2H9²+ + 2e¯ → Hg2²
Hg²+ + 2e
Ag* + e¯ → Ag
Hg,
→ 2Br
1.087
Zn²+ + 2e¯ → Zn
-0.7618
2+
0.920
2H2O + 2e¯ → H2 + 20H-
Cr2+ + 2e¯ → Cr
-0.8277
→ Hg
0.851
-0.913
0.7996
Al³+ + 3e¯ –→ Al
-1.662
2+
+ 2e
→ 2Hg
0.7973
Be2+ + 2e¯ → Be
-1.847
Fe+ + e → Fe2+
0.771
Н, + 2е — 2H
-2.23
MnO4 + e¯ → MnO4²-
0.558
Mg²+ + 2e¯ –→ Mg
- 2.372
I3 + 2e¯ -→ 3I¯
0.536
Na* + e –→ Na
-2.71
I2 + 2e
→ 21-
0.5355
Ca2+ + 2e¯ → Ca
- 2.868
Cu* + e¯ –→ Cu
0.521
Lit + e → Li
- 3.04
O2 + 2H2O + 4e¯ → 40H-
Cu²+ + 2e
0.401
→ Cu
0.3419
Hg,Cl, + 2e¯ → 2Hg + 2CI-
0.26828
AgCl + e¯ → Ag + Cl¯
0.22233
True
O False
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
