• An unknown acid (HA) was titrated with NAOH(aq). •The following titration curve was constructed using the laboratory data. You will use this titration curve to answer the questions related to this problem. • Assume that all laboratory work was completed at 298 K. 14 12 10 8 6. 4. 2. 4. 6. 8. 10 12 14 16 18 20 22 24 26 28 30 Volume of Titrant/ mL show/mention it Click on the equivalence point for this titration.
• An unknown acid (HA) was titrated with NAOH(aq). •The following titration curve was constructed using the laboratory data. You will use this titration curve to answer the questions related to this problem. • Assume that all laboratory work was completed at 298 K. 14 12 10 8 6. 4. 2. 4. 6. 8. 10 12 14 16 18 20 22 24 26 28 30 Volume of Titrant/ mL show/mention it Click on the equivalence point for this titration.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter26: Determination Of The Solubility Product Of Ba(io3)2
Section: Chapter Questions
Problem 3ASA
Related questions
Question
100%
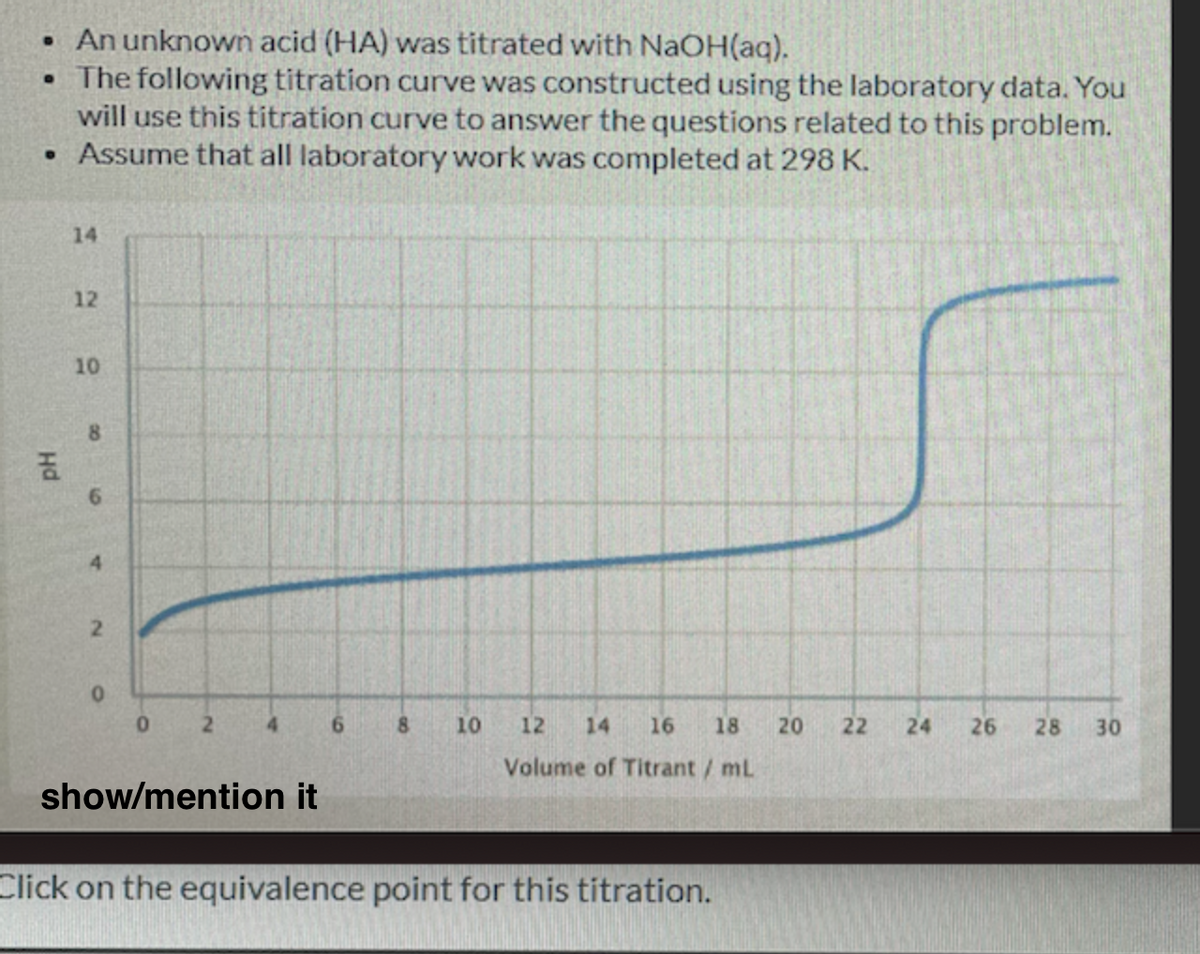
Transcribed Image Text:• An unknown acid (HA) was titrated with NAOH(aq).
•The following titration curve was constructed using the laboratory data. You
will use this titration curve to answer the questions related to this problem.
• Assume that all laboratory work was completed at 298 K.
14
12
10
8
6.
4.
2.
4.
6.
8.
10
12
14
16
18
20
22
24
26
28
30
Volume of Titrant/ mL
show/mention it
Click on the equivalence point for this titration.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole