Hint: No calculations are required. For the reaction CO:(g) + H2(g)CO(g) + H20(g) AH° = 41.2 kJ and AS° = 42.1 J/K At standard conditions, this reaction would be product favored O at relatively high temperatures. at no temperature. at relatively low temperatures. at all temperatures.
Hint: No calculations are required. For the reaction CO:(g) + H2(g)CO(g) + H20(g) AH° = 41.2 kJ and AS° = 42.1 J/K At standard conditions, this reaction would be product favored O at relatively high temperatures. at no temperature. at relatively low temperatures. at all temperatures.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section18.5: Entropy Changes And Spontaneity
Problem 3RC
Related questions
Question
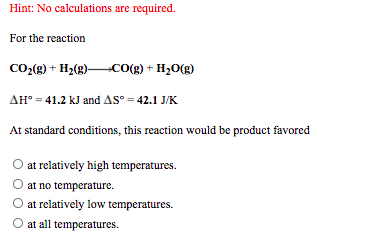
Transcribed Image Text:Hint: No calculations are required.
For the reaction
CO:(g) + H2(g)CO(g) + H20(g)
AH° = 41.2 kJ and AS° = 42.1 J/K
At standard conditions, this reaction would be product favored
O at relatively high temperatures.
at no temperature.
at relatively low temperatures.
at all temperatures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning