one of the following is not applicable on Mohr method of O a. dichromate might be used to prepare the indicator O b. silver complex of the indicator is more favored in precipitation than silver bromide O c. it does not work in acidic medium O d. Ksp of silver halidel is lower than Ksp of silverhalide2 if silver halide 2 is more water soluble Next page
one of the following is not applicable on Mohr method of O a. dichromate might be used to prepare the indicator O b. silver complex of the indicator is more favored in precipitation than silver bromide O c. it does not work in acidic medium O d. Ksp of silver halidel is lower than Ksp of silverhalide2 if silver halide 2 is more water soluble Next page
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.34QAP
Related questions
Question
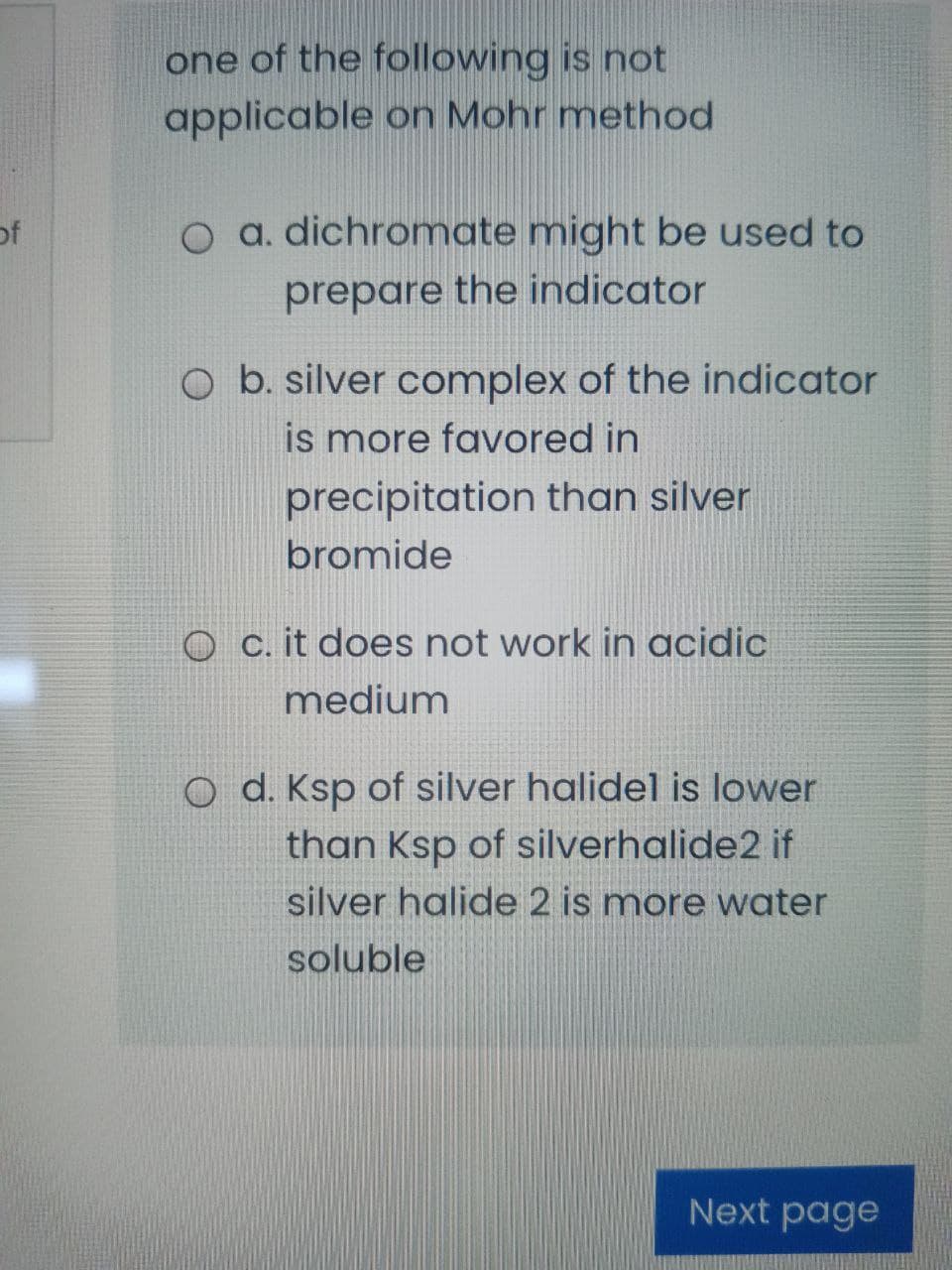
Transcribed Image Text:one of the following is not
applicable on Mohr method
of
O a. dichromate might be used to
prepare the indicator
O b. silver complex of the indicator
is more favored in
precipitation than silver
bromide
O c. it does not work in acidic
medium
O d. Ksp of silver halidel is lower
than Ksp of silverhalide2 if
silver halide 2 is more water
soluble
Next page
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning