If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 3 significant digits. A chem signs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential N2(9)+4 H,O(1)+4e N,H4(aq)+4OH (аq) E. =-1.16 V red Br,(1)+2e 2 Br (aq) E =+1.065 V red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Ox10 Write a balanced equation for the half-reaction that ? happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to Yes calculate the cell voltage under standard No onditions?
If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 3 significant digits. A chem signs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential N2(9)+4 H,O(1)+4e N,H4(aq)+4OH (аq) E. =-1.16 V red Br,(1)+2e 2 Br (aq) E =+1.065 V red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Ox10 Write a balanced equation for the half-reaction that ? happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to Yes calculate the cell voltage under standard No onditions?
Chapter25: Instruments For Optical Spectrometry
Section: Chapter Questions
Problem 25.21QAP
Related questions
Question
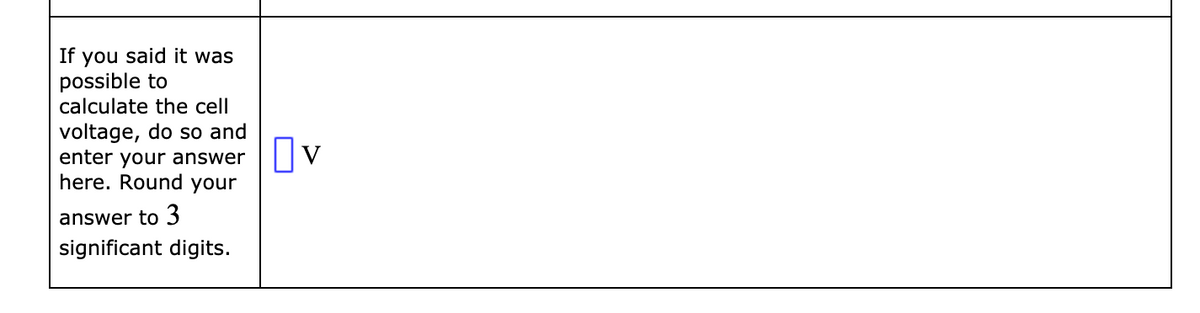
Transcribed Image Text:If you said it was
possible to
calculate the cell
voltage, do so and
enter your answer
here. Round your
answer to 3
significant digits.
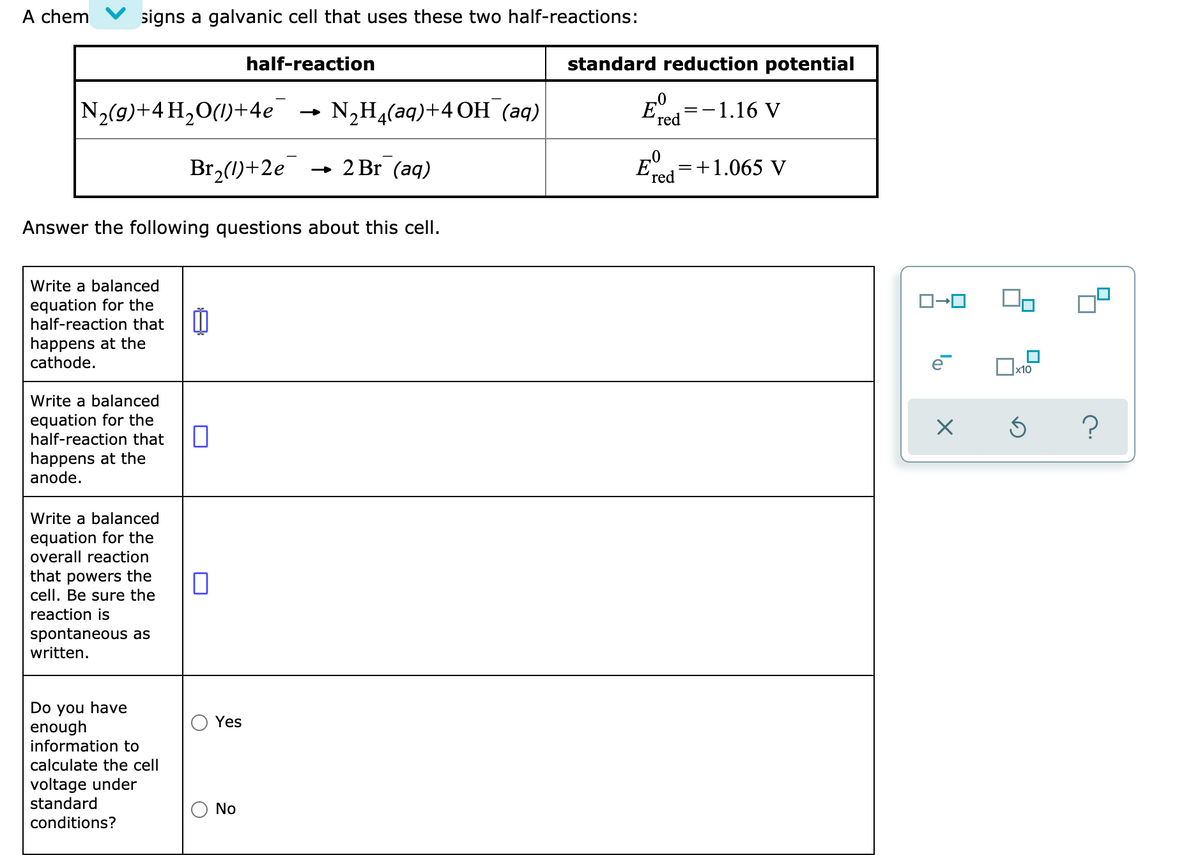
Transcribed Image Text:A chem
signs a galvanic cell that uses these two half-reactions:
half-reaction
standard reduction potential
N2(9)+4 H,O(1)+4e
N,H4(aq)+4OH (аq)
E.
=-1.16 V
red
Br,(1)+2e
2 Br (aq)
E
=+1.065 V
red
Answer the following questions about this cell.
Write a balanced
equation for the
half-reaction that
happens at the
cathode.
Ox10
Write a balanced
equation for the
half-reaction that
?
happens at the
anode.
Write a balanced
equation for the
overall reaction
that powers the
cell. Be sure the
reaction is
spontaneous as
written.
Do you have
enough
information to
Yes
calculate the cell
voltage under
standard
No
onditions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
