* 00 Consider the following reaction: CH,COOH(aq) +OH (aq) → CH;COO (aq) +H,0(1) 1st attempt See Periodic Table How much 6.01 MNAOH must be added to 510.0 mL of a buffer that is 0.0215 Macetic acid and 0.0265 M sodium acetate to raise the pH to 5.75? 4 OF 10 QUESTIONS COMPLETED < 02/10 > SUBMIT ANSWER 9 Type here to search 11:57 AM 71°F Sunny 11/1/2021 Lenovo DO DOLBY ATMOS SPEAKER SYSTEM Esc -D F2 +D F3 Delete FnLock F1 F4 F5 F7 F8 F11 F12 Insert & * Backspace 8 4. 5. 3. Tab A G Enter CapsLk H. Shift B. AS + PgUp Alt Ctrl Fn Alt Home tan OFF NO/ :- EXE (-)
* 00 Consider the following reaction: CH,COOH(aq) +OH (aq) → CH;COO (aq) +H,0(1) 1st attempt See Periodic Table How much 6.01 MNAOH must be added to 510.0 mL of a buffer that is 0.0215 Macetic acid and 0.0265 M sodium acetate to raise the pH to 5.75? 4 OF 10 QUESTIONS COMPLETED < 02/10 > SUBMIT ANSWER 9 Type here to search 11:57 AM 71°F Sunny 11/1/2021 Lenovo DO DOLBY ATMOS SPEAKER SYSTEM Esc -D F2 +D F3 Delete FnLock F1 F4 F5 F7 F8 F11 F12 Insert & * Backspace 8 4. 5. 3. Tab A G Enter CapsLk H. Shift B. AS + PgUp Alt Ctrl Fn Alt Home tan OFF NO/ :- EXE (-)
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 6ALQ: Sketch a pH curve for the titration of a weak acid (HA) with a strong base (NaOH). List the major...
Related questions
Question
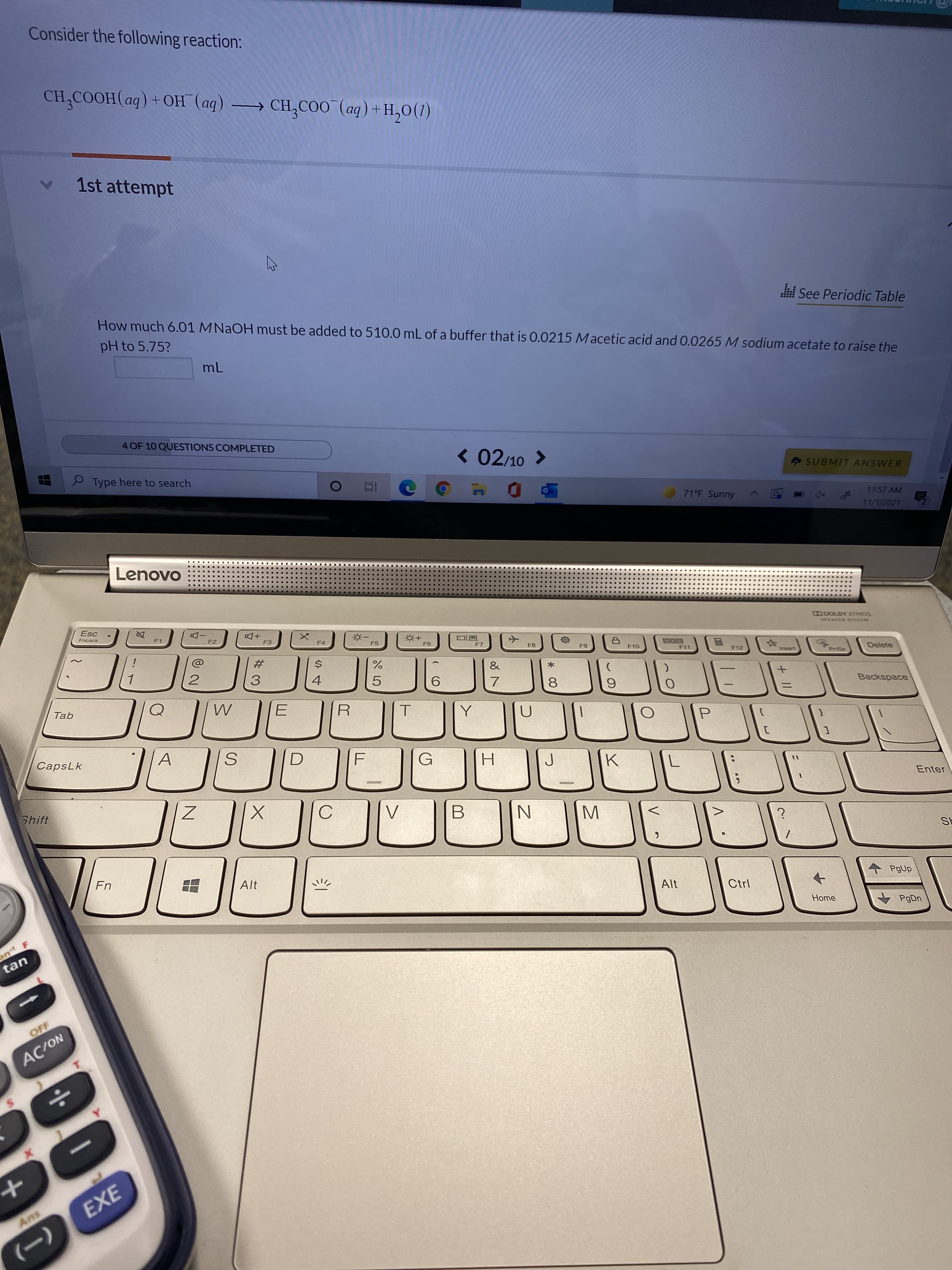
Transcribed Image Text:* 00
Consider the following reaction:
CH,COOH(aq) +OH (aq) → CH;COO (aq) +H,0(1)
1st attempt
See Periodic Table
How much 6.01 MNAOH must be added to 510.0 mL of a buffer that is 0.0215 Macetic acid and 0.0265 M sodium acetate to raise the
pH to 5.75?
4 OF 10 QUESTIONS COMPLETED
< 02/10 >
SUBMIT ANSWER
9 Type here to search
11:57 AM
71°F Sunny
11/1/2021
Lenovo
DO DOLBY ATMOS
SPEAKER SYSTEM
Esc
-D
F2
+D
F3
Delete
FnLock
F1
F4
F5
F7
F8
F11
F12
Insert
&
*
Backspace
8
4.
5.
3.
Tab
A
G
Enter
CapsLk
H.
Shift
B.
AS
+ PgUp
Alt
Ctrl
Fn
Alt
Home
tan
OFF
NO/
:-
EXE
(-)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
