HA is a weak acid. Which equilibrium corresponds to the equilibrium constant Kp for A ? а. А (aq) + ОН - (aq) НОА 2- (aq) b. "A "aq) + H2О () НА (ag) + ОН (aq) С. НА (aq) + ОН - (aq) H20 (1) + H+ (aq) d. "A (aq) + Hз0 + (aq) НА (aq) + H20 () е. НА (aq) + H20 () — Н2А + (аq) + ОН (аq)
HA is a weak acid. Which equilibrium corresponds to the equilibrium constant Kp for A ? а. А (aq) + ОН - (aq) НОА 2- (aq) b. "A "aq) + H2О () НА (ag) + ОН (aq) С. НА (aq) + ОН - (aq) H20 (1) + H+ (aq) d. "A (aq) + Hз0 + (aq) НА (aq) + H20 () е. НА (aq) + H20 () — Н2А + (аq) + ОН (аq)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 16PS: Several acids are listed here with their respective equilibrium constants: HF(aq) + H2O() H3O+(aq)...
Related questions
Question
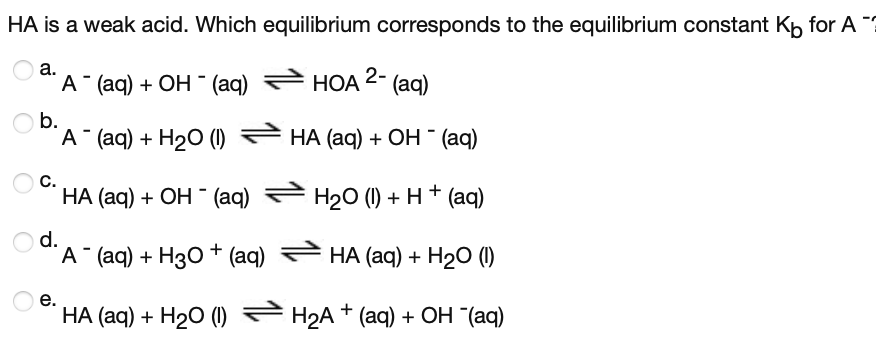
Transcribed Image Text:HA is a weak acid. Which equilibrium corresponds to the equilibrium constant Kp for A ?
а.
А (aq) + ОН - (aq)
НОА 2-
(aq)
b.
"A "aq) + H2О ()
НА (ag) + ОН (aq)
С.
НА (aq) + ОН - (aq)
H20 (1) + H+ (aq)
d.
"A (aq) + Hз0 + (aq)
НА (aq) + H20 ()
е.
НА (aq) + H20 () — Н2А + (аq) + ОН (аq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
