6b) Below are VSEPR diagrams representing fragments of the molecule from Lewis structure above Question 6; each diagram focuses on one atom and how atoms immediately surrounding it are positioned. Select all the fragments that are properly drawn. Note: fragments can be oriented in any direction as long as ALL atorms within the fragment are properly positioned with respect to one onother. C1 H N H NC, H-C-H a. b. C. d. O Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation. SHOW MORE V a. b b. C. d d. None of them are properly drawn 6c) Make sure you have correctly assigned all the electron pair geometries in the previous question and think about how a specific hybridization corresponds with a specific electron pair geometry when doing this matching game. Drag and drop options on the right-hand side and submit. For keyboard navigation. SHOW MORE V ci sp3 N C2 sp C sp o N1 sp2 N sp3 0 sp3 C 01 sp N sp2 0 sp2 C II II Questions 6a, b, c, d is finked to one another and focus on the molecule shown below. O: H C2 H. I-Z:
6b) Below are VSEPR diagrams representing fragments of the molecule from Lewis structure above Question 6; each diagram focuses on one atom and how atoms immediately surrounding it are positioned. Select all the fragments that are properly drawn. Note: fragments can be oriented in any direction as long as ALL atorms within the fragment are properly positioned with respect to one onother. C1 H N H NC, H-C-H a. b. C. d. O Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation. SHOW MORE V a. b b. C. d d. None of them are properly drawn 6c) Make sure you have correctly assigned all the electron pair geometries in the previous question and think about how a specific hybridization corresponds with a specific electron pair geometry when doing this matching game. Drag and drop options on the right-hand side and submit. For keyboard navigation. SHOW MORE V ci sp3 N C2 sp C sp o N1 sp2 N sp3 0 sp3 C 01 sp N sp2 0 sp2 C II II Questions 6a, b, c, d is finked to one another and focus on the molecule shown below. O: H C2 H. I-Z:
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
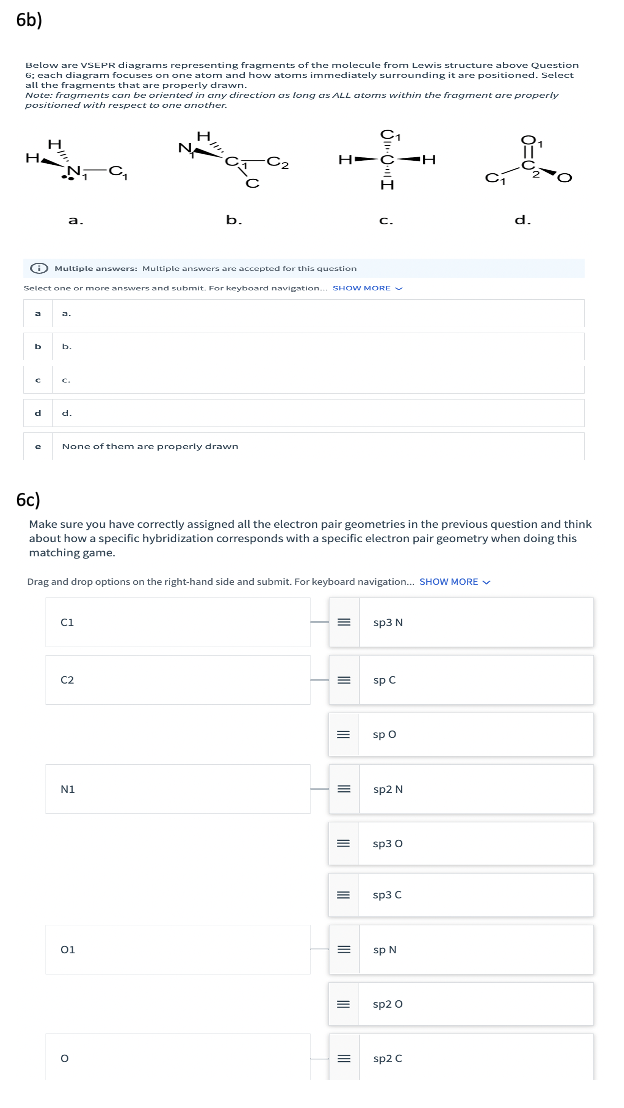
Transcribed Image Text:6b)
Below are VSEPR diagrams representing fragments of the molecule from Lewis structure above Question
6; each diagram focuses on one atom and how atoms immediately surrounding it are positioned. Select
all the fragments that are properly drawn.
Note: fragments can be oriented in any direction as long as ALL atorms within the fragment are properly
positioned with respect to one onother.
C1
H
N
H
NC,
H-C-H
a.
b.
C.
d.
O Multiple answers: Multiple answers are accepted for this question
Select one or more answers and submit. For keyboard navigation. SHOW MORE V
a.
b
b.
C.
d
d.
None of them are properly drawn
6c)
Make sure you have correctly assigned all the electron pair geometries in the previous question and think
about how a specific hybridization corresponds with a specific electron pair geometry when doing this
matching game.
Drag and drop options on the right-hand side and submit. For keyboard navigation. SHOW MORE V
ci
sp3 N
C2
sp C
sp o
N1
sp2 N
sp3 0
sp3 C
01
sp N
sp2 0
sp2 C
II
II
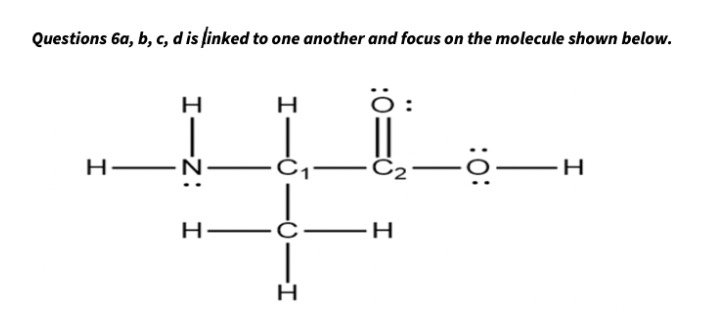
Transcribed Image Text:Questions 6a, b, c, d is finked to one another and focus on the molecule shown below.
O:
H
C2
H.
I-Z:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning