truct click submit at the end. DQuestion 40 How many grams are in 5.19 x 1023 molecules of carbon disulfide? « Previous No new data to save. Last checked at 9 46 082 esc F1 02 F3 F2 %23
truct click submit at the end. DQuestion 40 How many grams are in 5.19 x 1023 molecules of carbon disulfide? « Previous No new data to save. Last checked at 9 46 082 esc F1 02 F3 F2 %23
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 62QAP: An air bag is deployed by utilizing the following re tion the nitrogen gas produced inflates the air...
Related questions
Question
Transcribed Image Text:truct
click submit at the end.
DQuestion 40
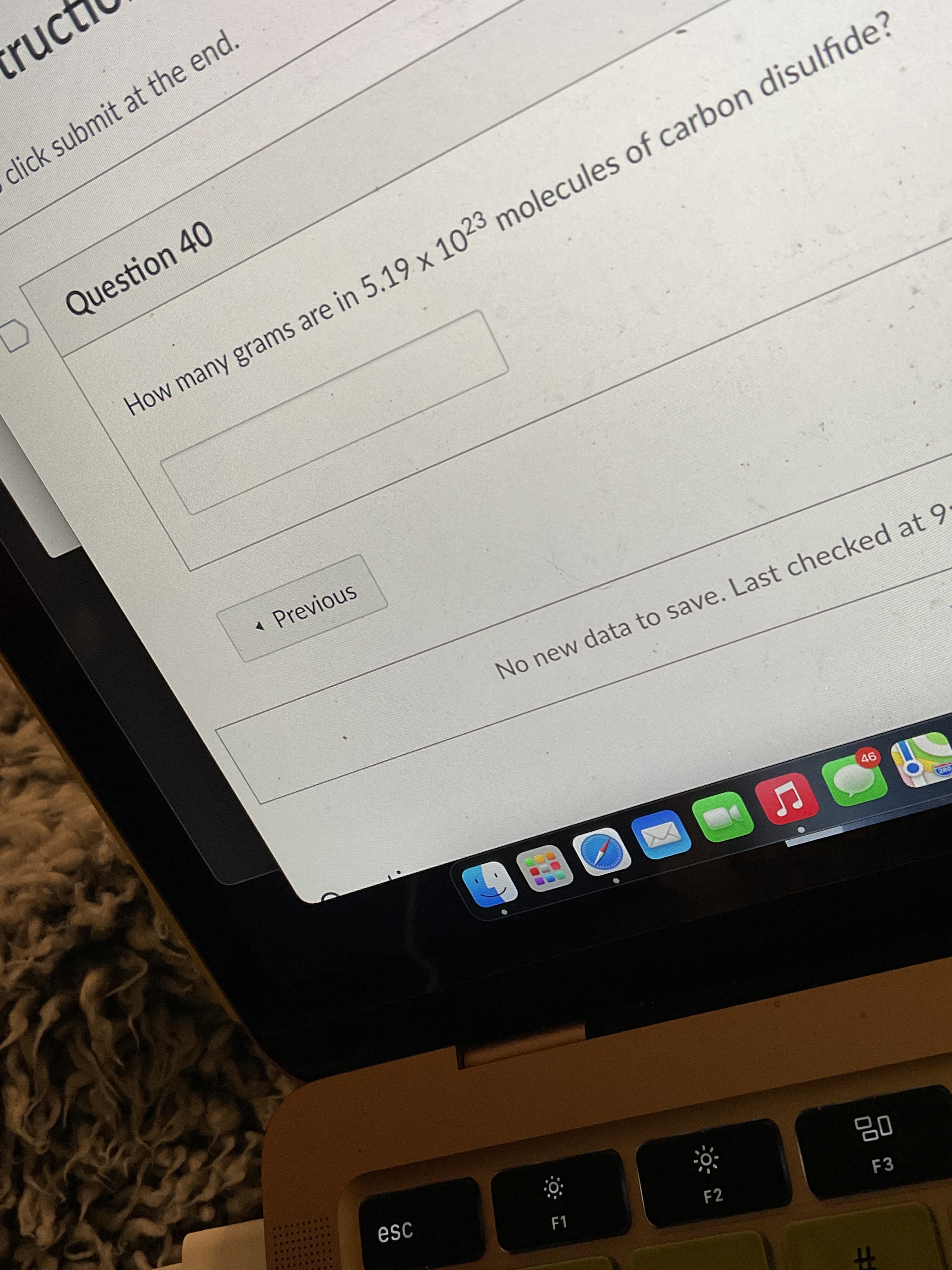
How many grams are in 5.19 x 1023 molecules of carbon disulfide?
« Previous
No new data to save. Last checked at 9
46
082
esc
F1
02
F3
F2
%23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
