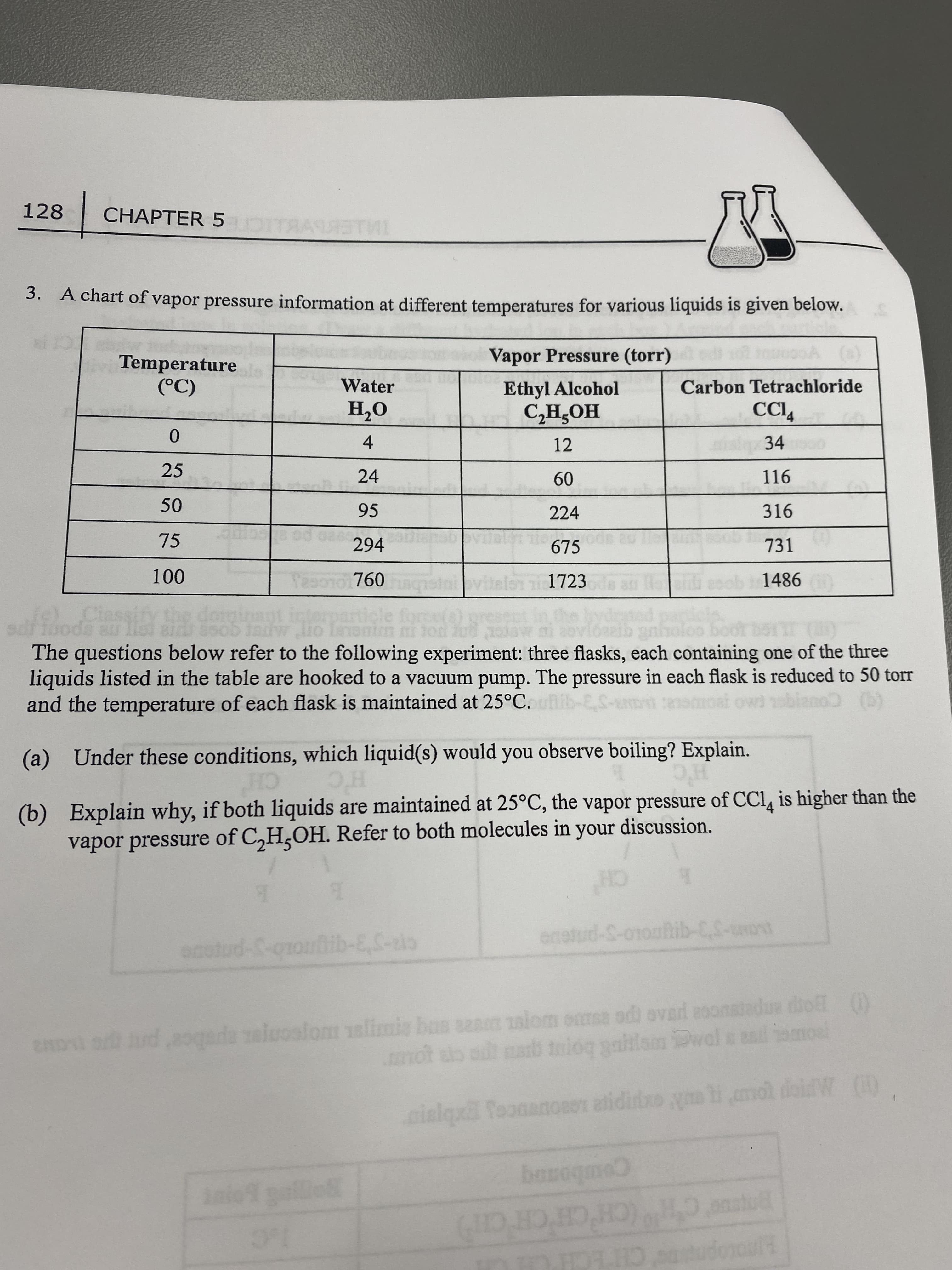
128 CHAPTER 5 3. A chart of vapor pressure information at different temperatures for various liquids is given below. Vapor Pressure (torr) Temperature Water Ethyl Alcohol Carbon Tetrachloride O'H 4. 24 (C) HOʻHƆ 12 34 0. 25 116 09 224 95 316 75 294 675 731 760 1723 1486 The questions below refer to the following experiment: three flasks, each containing one of the three liquids listed in the table are hooked to a vacuum pump. The pressure in each flask is reduced to 50 torr and the temperature of each flask is maintained at 25°C. blano (b) (a) Under these conditions, which liquid(s) would you observe boiling? Explain. (b) Explain why, if both liquids are maintained at 25°C, the vapor pressure of CCI, is higher than the vapor pressure of C,H,OH. Refer to both molecules in your discussion. CH H'C CH enstud-S-otoahib-CS-uot OBO 2ADI ud,8gada al sanm alom omss odi ovard asonatadue dhol () palom alimis bas Dwal s ad mot ab at a tmioq gaiilam Dwal inlqx ToonanoROT atidio ym li mol diW ) Comboanq anstul (CH'CHCH CH) 1.C HAOLOpree CHECH CH
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









