undergo the selected type of polymerization (hint: consider concepts such as electron donating group, electron withdrawing group, resonance, and hyperconjugation!): H. H : : H. ČH3 i. Radical Polymerization! R-O Initiation propagation Reason for monomer choice:
undergo the selected type of polymerization (hint: consider concepts such as electron donating group, electron withdrawing group, resonance, and hyperconjugation!): H. H : : H. ČH3 i. Radical Polymerization! R-O Initiation propagation Reason for monomer choice:
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter29: Organic Polymer Chemistry
Section: Chapter Questions
Problem 29.34P: Radical polymerization of styrene gives a linear polymer. Radical polymerization of a mixture of...
Related questions
Question
Consider each of the following monomers and predict if it would likely undergo radical, anionic, or cationic addition polymerisation. Then, in the space provided below, draw the electron pushing mechanism for the initiation and the first propagation steps of the monomer that you chose for each (the catalyst is provided for you). Then draw what the growing
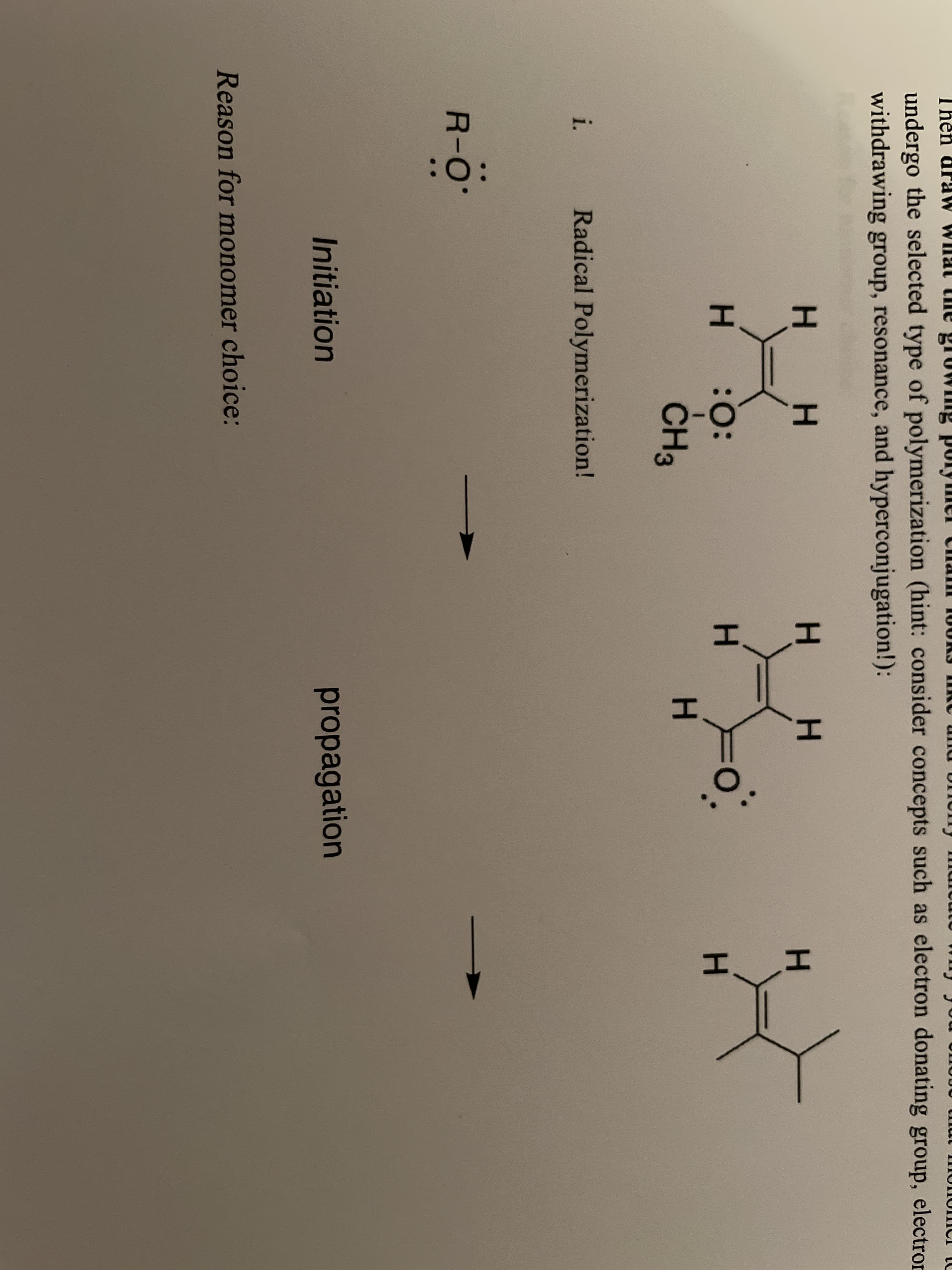
Transcribed Image Text:undergo the selected type of polymerization (hint: consider concepts such as electron donating group, electron
withdrawing group, resonance, and hyperconjugation!):
H.
H : :
H.
ČH3
i.
Radical Polymerization!
R-O
Initiation
propagation
Reason for monomer choice:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning