< (O %24 Complete the reactions of Sn(II) and Sn(IV), and be sure that the reactions are balanced. Do not include the phases (liquid, aqueous, etc.). If no reaction occurs, leave the products side of the equation blank. completed reaction: SnBr, + PbBr, –→ completed reaction: SnBr. + PbBr, → Select the statements that are true about the reactions. PbBr, is more stable than PbBr,. O The inert-pair effect renders Sn(II) as the more stable oxidation state of tin. The inert-pair effect renders Pb(II) as the more stable oxidation state of lead. OSn(IV) is the most stable oxidation state of tin. MacBook Air DD F7 F4 F5 F8 9- ししコ %23 3. 4. 5. 9. 7. 8. R. K. B. N
< (O %24 Complete the reactions of Sn(II) and Sn(IV), and be sure that the reactions are balanced. Do not include the phases (liquid, aqueous, etc.). If no reaction occurs, leave the products side of the equation blank. completed reaction: SnBr, + PbBr, –→ completed reaction: SnBr. + PbBr, → Select the statements that are true about the reactions. PbBr, is more stable than PbBr,. O The inert-pair effect renders Sn(II) as the more stable oxidation state of tin. The inert-pair effect renders Pb(II) as the more stable oxidation state of lead. OSn(IV) is the most stable oxidation state of tin. MacBook Air DD F7 F4 F5 F8 9- ししコ %23 3. 4. 5. 9. 7. 8. R. K. B. N
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 26Q: In theory, most metals should easily corrode in air. Why? A group of metals called the noble metals...
Related questions
Question
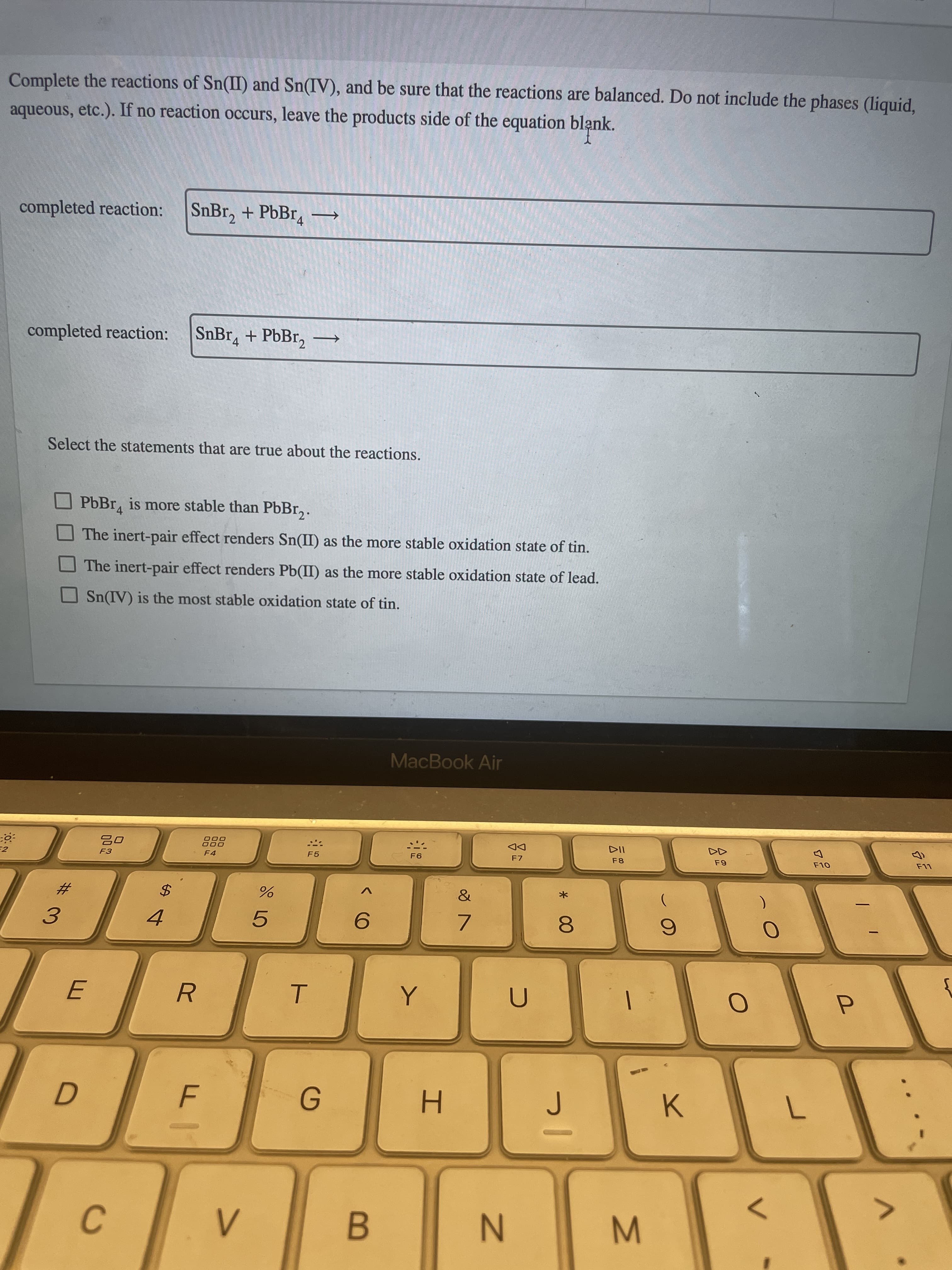
Complete the reactions of Sn(II) and Sn(IV), and be sure that the reactions are balanced. Do not include the phases (liquid, aqueous, etc.). If no reaction occurs, leave the products side of the equation blank.
completed reaction: SnBr_{2} + PbBr_{4⟶
completed reaction:
SnBr_{4} + PbBr_{2}⟶
Select the statements that are true about the reactions.
PbBr4 is more stable than PbBr2.
The inert‑pair effect renders Sn(II) as the more stable oxidation state of tin.
The inert‑pair effect renders Pb(II) as the more stable oxidation state of lead.
Sn(IV) is the most stable oxidation state of tin.
Transcribed Image Text:< (O
%24
Complete the reactions of Sn(II) and Sn(IV), and be sure that the reactions are balanced. Do not include the phases (liquid,
aqueous, etc.). If no reaction occurs, leave the products side of the equation blank.
completed reaction:
SnBr, + PbBr, –→
completed reaction:
SnBr. + PbBr, →
Select the statements that are true about the reactions.
PbBr, is more stable than PbBr,.
O The inert-pair effect renders Sn(II) as the more stable oxidation state of tin.
The inert-pair effect renders Pb(II) as the more stable oxidation state of lead.
OSn(IV) is the most stable oxidation state of tin.
MacBook Air
DD
F7
F4
F5
F8
9-
ししコ
%23
3.
4.
5.
9.
7.
8.
R.
K.
B.
N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
