phpZzYKNd.png C-5x348 5) A reaction that involves the formation and removal of a water molecule is called dehydration synthesis. о-н15х463 One example is when two glucose molecules react as shown here: он 6 CH₂OH C-H7X413 CH₂OH CH₂OH CH₂OH OH t Кон 2 2-0 5×358 C=0|1x 799 1740 2315 2891 1790 OH OH HO H он на OH H OH OH OH + H₂O HO 799=953514/mol OH OH OH Identify the bonds broken and the bonds that are formed and calculate the energy involved in this reaction and indicate if the reaction is endothermic or exothermic. Bonds broken: water molecule Bonds formed Bonds formed: Glycosidie band https://bio.libretexts.org/@api/deki/files/29008/figure-03-01-01.jpeg?revision=1 CH₂OH 2 03 H 3 1₂0 H Glueuse bonds have a bond on thepy of 9535 kJ/w/, and maltose (tive glucuses reated) have these plus a glycosidre band meaning 27 in relasiny Wre energy stan
phpZzYKNd.png C-5x348 5) A reaction that involves the formation and removal of a water molecule is called dehydration synthesis. о-н15х463 One example is when two glucose molecules react as shown here: он 6 CH₂OH C-H7X413 CH₂OH CH₂OH CH₂OH OH t Кон 2 2-0 5×358 C=0|1x 799 1740 2315 2891 1790 OH OH HO H он на OH H OH OH OH + H₂O HO 799=953514/mol OH OH OH Identify the bonds broken and the bonds that are formed and calculate the energy involved in this reaction and indicate if the reaction is endothermic or exothermic. Bonds broken: water molecule Bonds formed Bonds formed: Glycosidie band https://bio.libretexts.org/@api/deki/files/29008/figure-03-01-01.jpeg?revision=1 CH₂OH 2 03 H 3 1₂0 H Glueuse bonds have a bond on thepy of 9535 kJ/w/, and maltose (tive glucuses reated) have these plus a glycosidre band meaning 27 in relasiny Wre energy stan
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.75E
Related questions
Question
I tried to answer it, but I'm not sure if it is right, please help!

Transcribed Image Text:phpZzYKNd.png
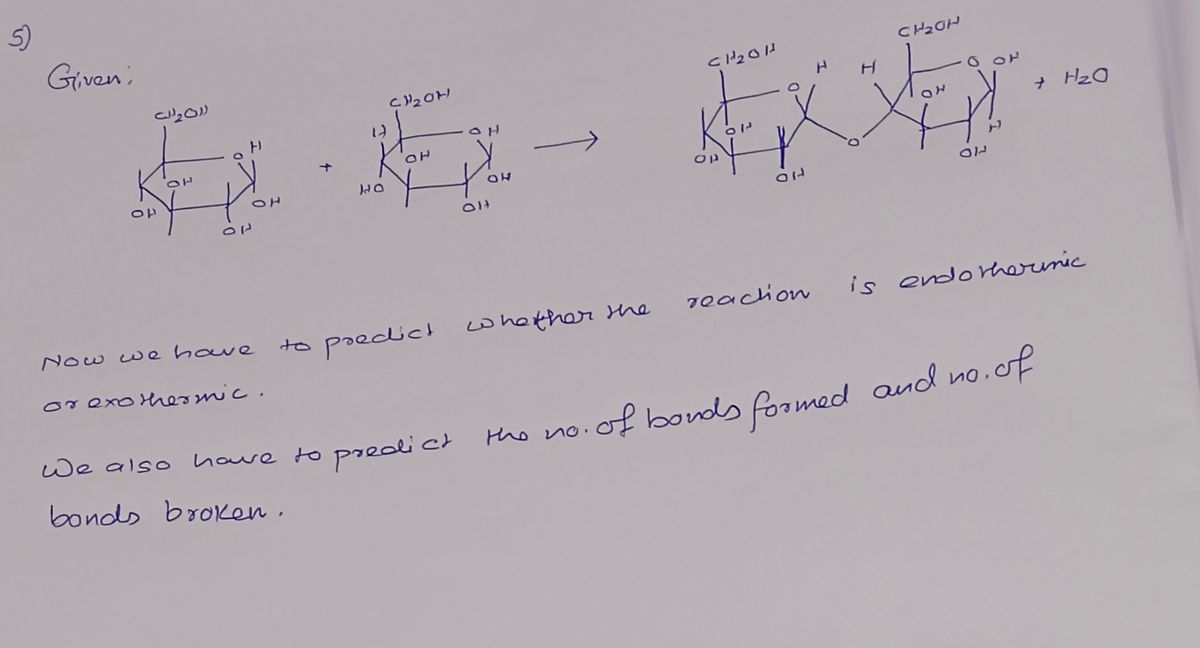
C-5x348 5) A reaction that involves the formation and removal of a water molecule is called dehydration synthesis.
о-н15х463
One example is when two glucose molecules react as shown here:
он 6
CH₂OH
C-H7X413
CH₂OH
CH₂OH
CH₂OH
OH
t
Кон
2
2-0 5×358
C=0|1x 799
1740
2315
2891
1790
OH
OH HO
H
он на
OH
H
OH
OH
OH
+ H₂O
HO
799=953514/mol OH
OH
OH
Identify the bonds broken and the bonds that are formed and calculate the energy involved in this reaction and
indicate if the reaction is endothermic or exothermic.
Bonds broken: water molecule
Bonds formed
Bonds formed: Glycosidie band
https://bio.libretexts.org/@api/deki/files/29008/figure-03-01-01.jpeg?revision=1
CH₂OH 2
03
H 3
1₂0
H
Glueuse bonds have a bond
on thepy
of 9535 kJ/w/, and
maltose (tive glucuses reated) have these plus a glycosidre band meaning
27
in relasiny
Wre
energy
stan
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning